Abstract
To avoid innate sensing and immune control, human immunodeficiency virus type 1 (HIV-1) has to prevent the accumulation of viral complementary DNA species. Here, we show that the late HIV-1 accessory protein Vpu hijacks DNA repair mechanisms to promote degradation of nuclear viral cDNA in cells that are already productively infected. Vpu achieves this by interacting with RanBP2–RanGAP1*SUMO1–Ubc9 SUMO E3-ligase complexes at the nuclear pore to reprogramme promyelocytic leukaemia protein nuclear bodies and reduce SUMOylation of Bloom syndrome protein, unleashing end degradation of viral cDNA. Concomitantly, Vpu inhibits RAD52-mediated homologous repair of viral cDNA, preventing the generation of dead-end circular forms of single copies of the long terminal repeat and permitting sustained nucleolytic attack. Our results identify Vpu as a key modulator of the DNA repair machinery. We show that Bloom syndrome protein eliminates nuclear HIV-1 cDNA and thereby suppresses immune sensing and proviral hyper-integration. Therapeutic targeting of DNA repair may facilitate the induction of antiviral immunity and suppress proviral integration replenishing latent HIV reservoirs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the finding of this study are available from the corresponding author on request. Complete western blot images in the manuscript are provided in the source data. Correspondence and requests for materials should be addressed to F.K. Source data are provided with this paper.
References
Craigie, R. & Bushman, F. D. HIV DNA integration. Cold Spring Harb. Perspect. Med. 2, a006890 (2012).
Brégnard, C., Benkirane, M. & Laguette, N. DNA damage repair machinery and HIV escape from innate immune sensing. Front. Microbiol. 5, 176 (2014).
Mamede, J. I., Cianci, G. C., Anderson, M. R. & Hope, T. J. Early cytoplasmic uncoating is associated with infectivity of HIV-1. Proc. Natl Acad. Sci. USA 114, E7169–E7178 (2017).
Towers, G. J. & Noursadeghi, M. Interactions between HIV-1 and the cell-autonomous innate immune system. Cell Host Microbe 16, 10–18 (2014).
Yan, N., Regalado-Magdos, A. D., Stiggelbout, B., Lee-Kirsch, M. A. & Lieberman, J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat. Immunol. 11, 1005–1013 (2010).
Ahn, J. et al. HIV-1 Vpr loads uracil DNA glycosylase-2 onto DCAF1, a substrate recognition subunit of a cullin 4A-ring E3 ubiquitin ligase for proteasome-dependent degradation. J. Biol. Chem. 285, 37333–37341 (2010).
Hrecka, K. et al. HIV-1 and HIV-2 exhibit divergent interactions with HLTF and UNG2 DNA repair proteins. Proc. Natl Acad. Sci. USA 113, E3921–E3930 (2016).
Schröfelbauer, B., Yu, Q., Zeitlin, S. G. & Landau, N. R. Human immunodeficiency virus type 1 Vpr induces the degradation of the UNG and SMUG uracil-DNA glycosylases. J. Virol. 79, 10978–10987 (2005).
Craigie, R., Fujiwara, T. & Bushman, F. The IN protein of Moloney murine leukemia virus processes the viral DNA ends and accomplishes their integration in vitro. Cell 62, 829–837 (1990).
Katz, R. A., Merkel, G., Kulkosky, J., Leis, J. & Skalka, A. M. The avian retroviral IN protein is both necessary and sufficient for integrative recombination in vitro. Cell 63, 87–95 (1990).
Daniel, R. et al. Evidence that stable retroviral transduction and cell survival following DNA integration depend on components of the nonhomologous end joining repair pathway. J. Virol. 78, 8573–8581 (2004).
Willey, R. L., Maldarelli, F., Martin, M. A. & Strebel, K. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J. Virol. 66, 7193–7200 (1992).
Wildum, S., Schindler, M., Munch, J. & Kirchhoff, F. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J. Virol. 80, 8047–8059 (2006).
Neil, S. J. D., Zang, T. & Bieniasz, P. D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451, 425–430 (2008).
Van Damme, N. et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe 3, 245–252 (2008).
Ryan, E. L., Hollingworth, R. & Grand, R. J. Activation of the DNA damage response by RNA viruses. Biomolecules 6, 2 (2016).
Bennardo, N., Cheng, A., Huang, N. & Stark, J. M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4, e1000110 (2008).
Akyuz, N. et al. DNA substrate dependence of p53-mediated regulation of double-strand break repair. Mol. Cell. Biol. 22, 6306–6317 (2002).
Restle, A. et al. Dissecting the role of p53 phosphorylation in homologous recombination provides new clues for gain-of-function mutants. Nucleic Acids Res. 36, 5362–5375 (2008).
Keimling, M. et al. Functional characterization connects individual patient mutations in ataxia telangiectasia mutated (ATM) with dysfunction of specific DNA double-strand break-repair signaling pathways. FASEB J. 25, 3849–3860 (2011).
Sauter, D. et al. Differential regulation of NF-κB-mediated proviral and antiviral host gene expression by primate lentiviral Nef and Vpu proteins. Cell Rep. 10, 586–599 (2015).
Volcic, M. et al. NF-κB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res. 40, 181–195 (2012).
Laguette, N. et al. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 156, 134–145 (2014).
Hotter, D. et al. Primate lentiviruses use at least three alternative strategies to suppress NF-κB-mediated immune activation. PLoS Pathog. 13, e1006598 (2017).
Cooper, A. et al. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature 498, 376–379 (2013).
Kmiec, D. et al. Vpu-mediated counteraction of tetherin is a major determinant of HIV-1 interferon resistance. mBio 7, e00934-16 (2016).
Langer, S. et al. HIV-1 Vpu is a potent transcriptional suppressor of NF-κB-elicited antiviral immune responses. eLife 8, e41930 (2019).
Yamada, E. et al. Human-specific adaptations in Vpu conferring anti-tetherin activity are critical for efficient early HIV-1 replication in vivo. Cell Host Microbe 23, 110–120 (2018).
Kueck, T. & Neil, S. J. D. A cytoplasmic tail determinant in HIV-1 Vpu mediates targeting of tetherin for endosomal degradation and counteracts interferon-induced restriction. PLoS Pathog. 8, e1002609 (2012).
Margottin, F. et al. A novel human WD protein, h-βTrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell 1, 565–574 (1998).
Akari, H., Bour, S., Kao, S., Adachi, A. & Strebel, K. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor κB–dependent expression of antiapoptotic factors. J. Exp. Med. 194, 1299–1312 (2001).
Huertas, P. DNA resection in eukaryotes: deciding how to fix the break. Nat. Struct. Mol. Biol. 17, 11–16 (2010).
Maréchal, A. & Zou, L. RPA-coated single-stranded DNA as a platform for post-translational modifications in the DNA damage response. Cell Res. 25, 9–23 (2015).
Mukherjee, B., Tomimatsu, N. & Burma, S. Immunofluorescence-based methods to monitor DNA end resection. Methods Mol. Biol. 1292, 67–75 (2015).
Croteau, D. L., Popuri, V., Opresko, P. L. & Bohr, V. A. Human RecQ helicases in DNA repair, recombination, and replication. Annu. Rev. Biochem. 83, 519–552 (2014).
Nimonkar, A. V., Ozsoy, A. Z., Genschel, J., Modrich, P. & Kowalczykowski, S. C. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc. Natl Acad. Sci. USA 105, 16906–16911 (2008).
Hampp, S. et al. DNA damage tolerance pathway involving DNA polymerase ι and the tumor suppressor p53 regulates DNA replication fork progression. Proc. Natl Acad. Sci. USA 113, E4311–E4319 (2016).
Eladad, S. et al. Intra-nuclear trafficking of the BLM helicase to DNA damage-induced foci is regulated by SUMO modification. Hum. Mol. Genet. 14, 1351–1365 (2005).
Ouyang, K. J., Yagle, M. K., Matunis, M. J. & Ellis, N. A. BLM SUMOylation regulates ssDNA accumulation at stalled replication forks. Front. Genet. 4, 167 (2013).
Dellaire, G. & Bazett-Jones, D. P. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. BioEssays 26, 963–977 (2004).
Vancurova, M. et al. PML nuclear bodies are recruited to persistent DNA damage lesions in an RNF168-53BP1 dependent manner and contribute to DNA repair. DNA Repair 78, 114–127 (2019).
Vigan, R. & Neil, S. J. D. Separable determinants of subcellular localization and interaction account for the inability of group O HIV-1 Vpu to counteract tetherin. J. Virol. 85, 9737–9748 (2011).
Jäger, S. et al. Global landscape of HIV–human protein complexes. Nature 481, 365–370 (2012).
Saitoh, N. et al. In situ SUMOylation analysis reveals a modulatory role of RanBP2 in the nuclear rim and PML bodies. Exp. Cell Res. 312, 1418–1430 (2006).
Sauter, D. et al. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6, 409–421 (2009).
Werner, A., Flotho, A. & Melchior, F. The RanBP2/RanGAP1*SUMO1/Ubc9 complex is a multisubunit SUMO E3 ligase. Mol. Cell 46, 287–298 (2012).
Kilzer, J. M. et al. Roles of host cell factors in circularization of retroviral DNA. Virology 314, 460–467 (2003).
Shank, P. R. et al. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell 15, 1383–1395 (1978).
Chandramouly, G. et al. Small-molecule disruption of RAD52 rings as a mechanism for precision medicine in BRCA-deficient cancers. Chem. Biol. 22, 1491–1504 (2015).
Kan, Y., Batada, N. N. & Hendrickson, E. A. Human somatic cells deficient for RAD52 are impaired for viral integration and compromised for most aspects of homology-directed repair. DNA Repair 55, 64–75 (2017).
Lau, A., Kanaar, R., Jackson, S. P. & O’Connor, M. J. Suppression of retroviral infection by the RAD52 DNA repair protein. EMBO J. 23, 3421–3429 (2004).
Schaller, T. et al. HIV-1 capsid–cyclophilin interactions determine nuclear import pathway, integration targeting and replication efficiency. PLoS Pathog. 7, e1002439 (2011).
Di Nunzio, F. et al. Human nucleoporins promote HIV-1 docking at the nuclear pore, nuclear import and integration. PLoS ONE 7, e46037 (2012).
Zhang, R., Mehla, R. & Chauhan, A. Perturbation of host nuclear membrane component RanBP2 impairs the nuclear import of human immunodeficiency virus-1 preintegration complex (DNA). PLoS ONE 5, e15620 (2010).
Galão, R. P., Le Tortorec, A., Pickering, S., Kueck, T. & Neil, S. J. D. Innate sensing of HIV-1 assembly by tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe 12, 633–644 (2012).
Mohammadi, P. et al. 24 hours in the life of HIV-1 in a T cell line. PLoS Pathog. 9, e1003161 (2013).
Lama, J. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr. HIV Res. 1, 167–184 (2003).
Klimkait, T., Strebel, K., Hoggan, M. D., Martin, M. A. & Orenstein, J. M. The human immunodeficiency virus type 1-specific protein Vpu is required for efficient virus maturation and release. J. Virol. 64, 621–629 (1990).
König, R. et al. Global analysis of host–pathogen interactions that regulate early-stage HIV-1 replication. Cell 135, 49–60 (2008).
Zhong, S. et al. A role for PML and the nuclear body in genomic stability. Oncogene 18, 7941–7947 (1999).
Sohn, S.-Y. & Hearing, P. Adenovirus regulates sumoylation of Mre11–Rad50–Nbs1 components through a paralog-specific mechanism. J. Virol. 86, 9656–9665 (2012).
Boichuk, S., Hu, L., Makielski, K., Pandolfi, P. P. & Gjoerup, O. V. Functional connection between Rad51 and PML in homology-directed repair. PLoS ONE 6, e25814 (2011).
Lusic, M. et al. Proximity to PML nuclear bodies regulates HIV-1 latency in CD4+ T cells. Cell Host Microbe 13, 665–677 (2013).
Erdal, E., Haider, S., Rehwinkel, J., Harris, A. L. & McHugh, P. J. A prosurvival DNA damage-induced cytoplasmic interferon response is mediated by end resection factors and is limited by Trex1. Genes Dev. 31, 353–369 (2017).
Himmels, S.-F. & Sartori, A. A. Controlling DNA-end resection: an emerging task for ubiquitin and SUMO. Front. Genet. 7, 152 (2016).
González-Prieto, R., Cuijpers, S. A. G., Luijsterburg, M. S., van Attikum, H. & Vertegaal, A. C. O. SUMOylation and PARylation cooperate to recruit and stabilize SLX4 at DNA damage sites. EMBO Rep. 16, 512–519 (2015).
Kirby, T. J. & Lammerding, J. Emerging views of the nucleus as a cellular mechanosensor. Nat. Cell Biol. 20, 373–381 (2018).
Vermeire, J. et al. HIV triggers a cGAS-dependent, Vpu- and Vpr-regulated type I interferon response in CD4+ T cells. Cell Rep. 17, 413–424 (2016).
Levy, J. A., Virolainen, M. & Defendi, V. Human lymphoblastoid lines from lymph node and spleen. Cancer 22, 517–524 (1968).
Pedrazzi, G. Direct association of Bloom’s syndrome gene product with the human mismatch repair protein MLH1. Nucleic Acids Res. 29, 4378–4386 (2001).
Uhl, M. et al. Role of SIRT1 in homologous recombination. DNA Repair 9, 383–393 (2010).
Gole, B., Baumann, C., Mian, E., Ireno, C. I. & Wiesmüller, L. Endonuclease G initiates DNA rearrangements at the MLL breakpoint cluster upon replication stress. Oncogene 34, 3391–3401 (2015).
Obermeier, K. et al. Heterozygous PALB2 c.1592delT mutation channels DNA double-strand break repair into error-prone pathways in breast cancer patients. Oncogene 35, 3796–3806 (2016).
Lambert, S. & Lopez, B. S. Characterization of mammalian RAD51 double strand break repair using non-lethal dominant-negative forms. EMBO J. 19, 3090–3099 (2000).
Sauter, D. et al. Human tetherin exerts strong selection pressure on the HIV-1 group N Vpu protein. PLoS Pathog. 8, e1003093 (2012).
Herzer, K., Weyer, S., Krammer, P. H., Galle, P. R. & Hofmann, T. G. Hepatitis C virus core protein inhibits tumor suppressor protein promyelocytic leukemia function in human hepatoma cells. Cancer Res. 65, 10830–10837 (2005).
Collis, S. J., Swartz, M. J., Nelson, W. G. & DeWeese, T. L. Enhanced radiation and chemotherapy-mediated cell killing of human cancer cells by small inhibitory RNA silencing of DNA repair factors. Cancer Res. 63, 1550–1554 (2003).
Lallemand-Breitenbach, V. et al. Arsenic degrades PML or PML–RARα through a SUMO-triggered RNF4/ubiquitin-mediated pathway. Nat. Cell Biol. 10, 547–555 (2008).
Jacque, J.-M. & Stevenson, M. The inner-nuclear-envelope protein emerin regulates HIV-1 infectivity. Nature 441, 641–645 (2006).
Rao, X., Huang, X., Zhou, Z. & Lin, X. An improvement of the 2ΔΔCT method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinforma. Biomath. 3, 71–85 (2013).
Munir, S., Thierry, S., Subra, F., Deprez, E. & Delelis, O. Quantitative analysis of the time-course of viral DNA forms during the HIV-1 life cycle. Retrovirology 10, 87 (2013).
Acknowledgements
We thank D. Schloesser for technical help with the PLA experiments, V. Lallemand-Breitenbach for providing us with the antibody recognizing PML isoforms I–IV, M. Lusic for support and helpful discussions, and J. M. Stark for giving us the EJ5SceGFP construct for NHEJ measurements. The Quadro P6000 used for this research project was made available by the NVIDIA Corporation. L.W. was supported by grants from the German Research Foundation (grant no. DFG WI 3099/7, B05 in CRC 1279), European–German Space Agency (ESA/DLR) and German Ministry of Economy (BMWi, A0-10-IBER-2 funding grant no. 50WB1225). F.K. was supported by the DFG (grant no. KI 548/11-1; CRC 1279) and an Advanced ERC investigator grant (Anti-Virome). D.S., K.M.J.S. and F.K. are funded by the DFG priority program grant no. SPP1923 and T.G.H. is supported by CRC 1361. K.M.J.S. was supported by a Marie Skłodowska-Curie Individual Fellowship from the European Union’s Framework Program for Research and Innovation Horizon 2020 (2014–2020) under grant agreement no. 794803 (‘VIAR’) and the DFG (grant no. SP1600/4-1).
Author information
Authors and Affiliations
Contributions
M.V. performed and analysed most of the experiments. K.M.J.S., L.K., D.H. and D.S. contributed to additional assay work, image analysis and experimental expertise. C.M.S. performed the cloning. M.S. and T.S. contributed to the assays to analyse PML. N.J.A. contributed expertise and T.G.H. contributed materials and expertise. M.V., K.M.J.S., L.W. and F.K. wrote the manuscript. M.V., L.W. and F.K. supervised the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Primary DSB repair data and controls.
Primary DSB repair data and Role of DNA damage sensors. a, Primary FACS data for EGFP-based evaluation of DSB repair as in Fig. 1d. Evaluation of DSB repair frequency described in Methods section. b-c, Apoptosis induction and cell cycle distribution in Jurkat cells transfected with pHIV-1-NL4-3-env*-IRES-mCherry (HIV-1), pCMV-HA-I-SceI plus repair reporter plasmid HR-EGFP/5´EGFP. In parallel, split samples were treated with caspase inhibitor zVAD-fmk. Cells were fixed 48 h post-transfection with ethanol/acetone and stained with propidium iodide for DNA content analysis. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313. In c, samples in the same cell cycle phase were statistically analyzed. d, Extrachromosomal homologous repair frequency (Hom. repair freq.) in WTK1 cells transfected with pHIV-1-NL4-3-env*-IRES-mCherry, pCMV-HA-I-SceI plus repair plasmid HR-EGFP/3´EGFP for detection of HR and SSA (construct on top). Split samples were treated with zVAD-fmk. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, *p=0.0391. Control samples transfected with control plasmid set to 100 % (absolute mean value: 2 %). e-f, Apoptosis induction and cell cycle distribution in WTK1(HR/3′) cells 48 h after transfection with HIV-1 plus pCMV-HA-I-SceI. Bars represent means ± SEM, n=2 biologically independent experiments in duplicates (f) or triplicates (g). In (f) samples in the same cell cycle phase were statistically analyzed. g, Intrachromosomal homologous repair frequency in KMV(Δ/3′) cells transfected with HIV-1 plus pCMV-HA-I-SceI. Split samples were treated with and without Hygromycin B (60 µg/ml) 4 h post-transfection. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313, (absolute mean value: 0.2 %). Note the schematic presentation of the chromosomally integrated repair construct ΔEGFP/3´EGFP in KMV(Δ/3′) with a Hygromycin resistance cassette (blue bar) on top. h-l, Homologous repair frequency in WTK1(HR/3′) cells co-transfected with pHIV-1-NL4-3-env*-IRES-mCherry, pCMV-HA-I-SceI and shRNA to downregulate ATM (a), ATR (b), Nibrin (c), CtIP (d) and Ku70 (e) and corresponding empty vectors in the controls. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313 (absolute mean value: 0.04%). Lower panels show Western blots demonstrating efficiency of knockdowns. Two sided Wilcoxon matched-pairs test in b, d, g, h-l.
Extended Data Fig. 2 Vpu expression in different experimental settings and controls to exclude potentially confounding factors.
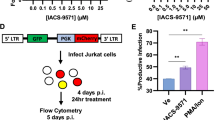
Vpu expression in different experimental settings and controls to exclude potentially confounding factors. a, Vpu titration. Evaluation of DSB repair frequencies in WTK1(HR/3′) cells transfected with increasing amounts of NL4-3 Vpu-AU1 expression plasmid. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313. Lower panels show Western blot demonstrating expression efficiency. b, Expression of WITO Vpu-AU1 in WTK1(HR/3′) cells transduced with pHIV-1-NL4-3 WITO Vpu-AU1 compared to cells transfected with pCG-WITO Vpu-AU1 expression vector or the pHIV-1-NL4-3 WITO Vpu-AU1 proviral construct. In the proviral construct vpu and env were separated and the NL-4-3 vpu replaced by an AU-1-tagged WITO vpu. 24 h post-transfection samples were analyzed by Western blot. c, Apoptosis and cell cycle analysis in WTK1(HR/3′) cells after transfection with pCG-NL4-3 Vpu-AU1 expression plasmid. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, statistical analysis was calculated for comparisons between different samples in the same cell cycle phase. d, Infection rates. Graph presenting mean values ± SD of p24 stained in CD4+ T cells from 3 donors shown in Fig. 3d. Right, primary flow cytometry data. Percentages of p24 positive cells in the live cell population (SSC/FSC gate) were calculated. e, Homologous repair, apoptosis and cell cycle in primary HIV-1 transfected CD4+ T cells. Cells were transfected with infectious (env+) HIV-1 CH058 wt, vpr* or vpu* constructs, together with repair reporter and meganuclease I-SceI expression plasmid. 48 h post-transfection samples were examined for repair frequencies or aceton/ethanol fixed and propidium iodide stained to evaluate cell cycle and apoptosis. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, *p=0.0152, **p=0.0022 (absolute mean value of homologous repair frequency was 0.3%). Two sided Wilcoxon matched-pairs test in a and e.
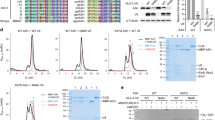
Extended Data Fig. 3 Analysis of mutant Vpu proteins and evaluation of known cellular Vpu targets.
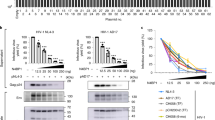
Analysis of mutant Vpu proteins and evaluation of known cellular Vpu targets. a, Sequence of WITO Vpu with mutated positions indicated. b, Western blot depicting expression of different WITO Vpu mutants from (a) and CH106 wt and its KKDQ mutant with the ER retention sequence. Experiment repeated 2 times with similar outcome. c, Homologous repair in WTK1(HR/3′) cells transfected with different mutants of WITO Vpu or CH106 and the CH106 KKDQ mutant with ER retention sequence. 48 h post transfection cells were analyzed by FACS to evaluate DSB repair frequencies. Bars represent means ± SEM, n=3 or 4 biologically independent experiments in triplicates, *p=0.0273, **p=0.0078, **p=0.0039 (absolute mean value: 0.7 %). Statistical evaluation shown for the comparison between wt WITO Vpu and the indicated Ala substitution mutants. d, BrdU assay to analyze DNA processing mediated by different WITO Vpu mutants. WTK1(HR/3′) WITO transfected cells were IR (2 Gy), pre-extracted, fixed and stained against BrdU. Data presents 4 independent experiments where each dot (n=14-18) presents mean value of 30-50 analyzed nuclei from one image view, ± SEM. *p=0.0166, **p=0.0012, **p=0.0085, ****p=0.0001. Black stars presenting comparison to the control, blue stars comparison to WITO Vpu. The lower panel shows representative BrdU and AU1 microscopic images. e, Homologous repair in WTK1(HR/3′) (β-TrCP). Cells were transfected with wt, vpr* or vpu* pHIV-1-NL4-3-env*-IRES-mCherry constructs together with expression plasmids for I-SceI and the DN form of β-TrCP. 48 h later cells were harvested for FACS analysis. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, **p=0.039 (absolute mean value: 0.1%). Lower panels show Western blot demonstrating overexpression of β-TrCP-DN. f, Immunoprecipitation (IP) of RAD52 1h after γ-irradiation (2Gy) of WTK1(HR/3′) cells transfected 24h before with NL4-3 Vpu expression plasmid or empty vector (Control). Graph presents mean values ± SD from two independent experiments. Note that RAD52 is difficult to detect by Western blotting unless it is concentrated for example by IP. Thus, most analyses had to rely on mRNA expression levels. g, Evaluation of RAD52 mRNA levels in SaOS cells treated as described in the legend Fig. 2i. Bars represent means ± SEM, n=4. Two sided Wilcoxon matched-pairs test in c, d, e, g.
Extended Data Fig. 4 Role of 53BP1 and cellular nucleases in HIV-1-mediated homologous repair regulation.
Role of 53BP1 and cellular nucleases in HIV-1-mediated homologous repair regulation. a, Detection of DSBs by 53BP1 foci. WTK1(HR/3′) cells transfected with wt or vpu* pHIV-1-NL4-3-env*-IRES-mCherry constructs were γ-irradiated (2Gy) or left untreated (0Gy), fixed and stained for 53BP1 at the indicated time points. Immunolabeled foci were scored by quantification in 150 nuclei each, n=3 biologically independent experiments. Maximum scores were set to 100% (absolute foci numbers for 53BP1: 7), bars represent means ±SD. The left panel shows representative microscopic images of 53BP1 foci. b, Role of DNase2, ERCC1 and FEN1 nucleases in HIV-1 mediated DNA repair. WTK1(HR/3′) cells were transfected with pHIV-1-NL4-3-env*-IRES-mCherry, I-SceI-HA and shRNA to silence DNase2, ERCC1 or FEN1. Bars represent means ± SEM, n=2 (DNase2, ERCC1) or n=4 (FEN1) biologically independent experiments in triplicates, *p=0.0313 (absolute mean values: Dnase2 2.2%, ERCC1 0.3% and FEN1 1.0%). Right three graphs presenting rtPCR analysis to show DNase2, ERCC1 and FEN1 knockdown efficiencies. Bars represent means ± SEM, n=4 biologically independent experiments, two sided unpaired t-test, ***p=0.0001. c, Homologous repair frequency in WTK1(HR/3′) cells 48 h after transfection with pHIV-1-NL4-3-env*-IRES-mCherry constructs containing intact or defective vpu genes, pCMV-HA-I-SceI and shRNA to silence SLX4. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313 (absolute mean value: 0.1%). d, Western blot analysis of WTK1(HR/3′) cells 48h after transfection with pHIV-1-NL4-3-env*-IRES-mCherry and pCMV-HA-I-SceI plasmids. The left panel shows examples of primary data and the right panel mean values ± SD of 3-6 independent experiments after quantification of band intensities using Bio-Rad ChemiDoc. Two sided Wilcoxon matched-pairs test in b (left three panels) and c.
Extended Data Fig. 5 Role of BLM and EXO1 in Vpu-mediated modulation of DNA end processing.
Role of BLM and EXO1 in Vpu-mediated modulation of DNA end processing. a, Representative images for immunofluorescence analysis of p-RPA and BrdU and b, knock-down efficiency of BLM and EXO1. c, p-RPA accumulation as a function of BLM. WTK1(HR/3′) cells were γ-irradiated (2Gy) 48h after transfection with wt or vpu* pHIV-1-NL4-3-env*-IRES-mCherry plus shRNA to silence BLM (BLMkd). 1h post-irradiation, samples were taken for p-RPA32 S4/8 and RPA Western Blot analysis. The right panel shows mean values (±SD) of p-RPA levels obtained in three independent experiments relative to control cells. d, p-RPA Western blot analysis in WTK1(HR/3′) cells transfected with wt or vpu* pHIV-1-NL4-3-env*-IRES-mCherry, together with shRNA against EXO1. 48 h after transfection cells were irradiated and taken for Western blotting. e, Expression of BLM and EXO1 in primary CD4+ T cells. CD4+ T cells isolated form two blood donors were either treated with IL-2 alone or stimulated with IL-2/PHA or IL-2 and CD3/CD28 magnetic beads or left untreated (U) for 3d when cells were harvested for Western blot analysis.
Extended Data Fig. 6 Effect of Vpu on PML-NBs. Role of BLM and EXO1 in Vpu-medidated modulation of DNA end processing.
Effect of Vpu on PML-NBs. Role of BLM and EXO1 in Vpu-mediated modulation of DNA end processing. a, Immunofluorescence analysis of Sp100 foci formation in WTK1(HR/3′) cells transfected with 1µg NL4-3, WITO Vpu or empty control. Cells were fixed at indicated time points. Data presents 3 independent experiments where each dot (n=6) represents mean value of 30-50 analyzed cells from one microscopic view; bars represent means ±SEM, *p=0.0313. Right panel, representative microscopic images. b, c, Immunofluorescence analysis of PML-NB and Sp100 foci in WTK1(HR/3′) cells transfected with wt or vpu* pHIV-1-CH058-env*-IRES-BFP constructs. Data present three independent experiments where each dot represents mean value of 30-50 analyzed cells from one microscopic view; bars represent means ±SEM, *p=0.0313. Right panel, representative microscopic images. Two sided Wilcoxon matched-pairs test in a-c.
Extended Data Fig. 7 Localization of Vpu at the nuclear membrane and effects on RanBP2-RanGAP1 proximity and function.
Localization of Vpu at the nuclear membrane and effects on RanBP2-RanGAP1 proximity and function. a, Subcellular protein fractionation performed in CD4+ T cells transfected with pCG-WITO Vpu-AU1 expression plasmid or empty control. Experiment conducted 2 times with similar outcome. b, Confocal microscopic localization analysis of Vpu and RanBP2. SaOS cells were transfected with 1 µg SIVcpz MB897 Vpu-AU1. 24 h later cells were fixed, permeabilized and costained against AU1-tagged Vpu and RanBP2. Graph on left presents the probability of Vpu following RanBP2 calculated from the images such as displayed on the right, bars represent means ± SEM, Two sided unpaired t- test here and in c, n=6, ****p=0.0001. Size bar = 5 µm. c, Primary CD4+ T cells were transfected with 0.5 µg of a vector expressing MB897 Vpu-AU1. Cells were permeabilized, stained with anti-AU1 and RanBP2 Ab and analyzed by confocal microscopy 24h post-transfection. Graph on left presents the probability of Vpu following RanBP2 calculated from the images such as displayed on the right, bars represent means ±SEM, n=5, ****p=0.0001. Size bar =5 µm. d, RanBP2 and RanGAP1 PLA in primary CD4+ T cells. Cells were transfected with 0.5 µg WITO Vpu-AU1 expression plasmid or empty vector control. Left, evaluation of RanBP2-RanGAP1 PLA foci per cell from two different donors, n=32, bars represent means ± SEM, two sided Mann-Whitney test, ****p=0.0001. Right, representative microscopic images. e, PLA to evaluate RanGAP1 and SUMO1 interaction in WTK1(HR/3′) cells transfected with WITO Vpu-AU1 expression plasmid or empty control. Left panel presenting evaluation of RanGAP1-SUMO PLA foci per cell, n=4 biologically independent experiments; two sided Wilcoxon matched-pairs test, mean values ± SEM, right panel presenting microscopic images. f, PLA assay to evaluate EXO1 and SUMO2/3 interaction in WTK1(HR/3′) cells transfected with WITO Vpu-AU1 expression plasmid or empty control. Left panel presenting evaluation of EXO1-SUMO2/3 foci per cell, n=2 biologically independent experiments, two sided Wilcoxon matched-pairs test (control n= 27, WITO n=20), mean values ± SEM, ****p=0.0001. Right panel presenting microscopic images.
Extended Data Fig. 8 Role of PIAS1, PIAS4 and RanBP2 SUMO ligases in BLM SUMOylation.
Role of PIAS1, PIAS4 and RanBP2 SUMO ligases in BLM SUMOylation. a, Role of PIAS1, PIAS4 and RanBP2 SUMO ligases in BLM SUMOylation. WTK1(HR/3′) cells were transfected with 1 µg WITO Vpu-AU1 or control plus 3 µg of shRNA to target PIAS1, PIAS4 and RanBP2. 24h later PLA was performed. Left, evaluation of BLM-SUMO2/3 foci per cell, n=3 biologically independent experiments where each dot (8-12) represents a mean from 30-50 cells analyzed from one microscopic view; bars represent means ± SEM, two sided Wilcoxon matched-pairs test, *p=0.0117, **p=0.002, **p=0.0039, **p=0.0078. Right, Western blots presenting knockdown of PIAS1, PIAS4 and RanBP2. Lower panels, representative microscopic images. b, WTK1(HR/3′) cells were transfected with 1 µg WITO Vpu-AU1 or control and stained against SUMO1, RanBP2 and RanGAP1 24h later. Intensity profiles including the corresponding DAPI (blue) staining in the presence (red) or absence (black) of WITO vpu were quantified using ImageJ. Displayed are the means (full lines) of 10µm profiles of 25 individually quantified and aligned cells including SEM (dotted line). Exemplary confocal images are shown next to the quantifications and the profile indicated by a white line.
Extended Data Fig. 9 Effect of Vpu and RAD52 or BLM on unintegrated viral cDNA, integration and superinfection in primary CD4+ T cells.
Effect of Vpu and RAD52 or BLM on unintegrated viral cDNA, integration and superinfection in primary CD4+ T cells. a-c, rtPCR analysis of 1-LTR, 2-LTR circles and integration in CD4+ T cells infected with wt or vpu* pHIV-1-NL4-3 in the presence or absence of a RAD52 inhibitor (5 µM 6-Hydroxy-DL-DOPA) 48 h post infection. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, (a) *p=0.0156, **p=0.0039, (b) **p=0.0078, (c) *p=0.0273, **p=0.0039. d, Propidium iodide staining of acetone/ethanol fixed cells and p24 stain of primary CD4+ T cells treated as in Fig. 7 (a–c). n=3, bars represent means ± SEM of 6 (apoptosis) or 4 measurements, **p=0.0022. e, Western blot analysis of p24 protein expression in CD4+ T cells infected with wt or vpu* pHIV-1-NL4-3. Experiment conducted two times with similar outcome. f-k, rtPCR analysis of 1-LTR, 2-LTR circles and integration in CD4+ T cells transduced with wt, vpu* or vpr* defective pHIV-1-NL4-3-env* constructs (f-h) or infected with replication-competent pHIV-1-NL4-3 in the presence of the protease inhibitor saquinavir (i-k). Bars represent means ± SEM from 6 (f,g), 5 (h), 3(i), 2(j, k) donors are shown. Analysis done 48 h post-infection/transduction. l, Western blot demonstrating BLM knockdown efficiency in Jurkat cells. Experiment conducted 2 times with similar outcome. m-o, rtPCR analysis of 1-LTR and 2-LTR circles and integration in BLM silenced Jurkat cells infected with wt or vpu* VSV-G pseudotyped pHIV-1-NL4-3. Bars represent means ± SEM, n=3 biologically independent experiments in triplicates, (m) *p=0.0156, **p=0.0039; (n) *p=0.0313, **p=0.0039, (o) **p=0.0039. Analysis done 48 h post-infection. p, Supernatants from m-o were taken to analyze infectious yields determined by β-galactosidase assay in TZM-bl cells. Bars represent means ± SEM, n=2 biologically independent experiments in triplicates, *p=0.0313. Two sided Wilcoxon matched-pairs test in a-d, m-p.
Extended Data Fig. 10 Effect of Vpu on HIV-1 superinfection in primary CD4+ T cells and BLM deficient cells.
Effect of Vpu on HIV-1 superinfection in primary CD4+ T cells and BLM deficient cells. a, Schematic presentation of the experimental design to measure and calculate HIV-1 superinfection. b, Primary flow cytometry data of CD4+ T cells treated as described in panel b; n=6. Calculated percentages of superinfected cells are shown between the dot plot panels. c, Primary flow cytometry data of BLM+/- cells treated as described in the legend to Fig. 6r.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Supplementary Table 1.
Supplementary Video 1
Video presenting colocalization between WITO Vpu and RanBP2 in transfected SaOS cells.
Supplementary Video 2
Video presenting colocalization between SIV Vpu and RanBP2 in transfected CD4+ T cells.
Supplementary Video 3
Video presenting colocalization between SIV Vpu and RanBP2 in transfected SaOS cells.
Source data
Source Data Fig. 1
Unprocessed western blots and gel.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Volcic, M., Sparrer, K.M.J., Koepke, L. et al. Vpu modulates DNA repair to suppress innate sensing and hyper-integration of HIV-1. Nat Microbiol 5, 1247–1261 (2020). https://doi.org/10.1038/s41564-020-0753-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0753-6
This article is cited by
-
ABRAXAS1 orchestrates BRCA1 activities to counter genome destabilizing repair pathways—lessons from breast cancer patients
Cell Death & Disease (2023)
-
N6-methyladenosine modification of the Aedes aegypti transcriptome and its alteration upon dengue virus infection in Aag2 cell line
Communications Biology (2022)
-
IFITM proteins promote SARS-CoV-2 infection and are targets for virus inhibition in vitro
Nature Communications (2021)