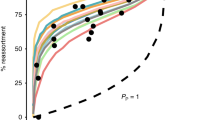
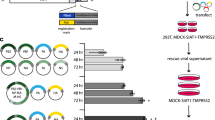
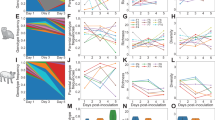
Abstract
Infection with a single influenza A virus (IAV) is only rarely sufficient to initiate productive infection. Instead, multiple viral genomes are often required in a given cell. Here, we show that the reliance of IAV on multiple infection can form an important species barrier. Namely, we find that avian H9N2 viruses representative of those circulating widely at the poultry–human interface exhibit acute dependence on collective interactions in mammalian systems. This need for multiple infection is greatly reduced in the natural host. Quantification of incomplete viral genomes showed that their complementation accounts for the moderate reliance on multiple infection seen in avian cells but not the added reliance seen in mammalian cells. An additional form of virus–virus interaction is needed in mammals. We find that the PA gene segment is a major driver of this phenotype and that both viral replication and transcription are affected. These data indicate that multiple distinct mechanisms underlie the reliance of IAV on multiple infection and underscore the importance of virus–virus interactions in IAV infection, evolution and emergence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
10x Genomics single-cell sequencing data are available on the GEO database with the accession number GSE135553. Other data are included as Source Data or are available from the corresponding author on reasonable request. Source data are provided with this paper.
Code availability
The code used for the 10x Genomics analysis is available at https://github.com/njacobs627/GFHK99_Multiplicity. The code used to run the agent-based model on IAV reassortment was reported previously7,8 and is also available at https://github.com/njacobs627/GFHK99_Multiplicity. Source data are provided with this paper.
References
Leeks, A., Sanjuan, R. & West, S. A. The evolution of collective infectious units in viruses. Virus Res. 265, 94–101 (2019).
Brooke, C. B. Population diversity and collective interactions during influenza virus infection. J. Virol. 91, e01164-17 (2017).
Sanjuan, R. Collective infectious units in viruses. Trends Microbiol. 25, 402–412 (2017).
Brooke, C. B., Ince, W. L., Wei, J., Bennink, J. R. & Yewdell, J. W. Influenza A virus nucleoprotein selectively decreases neuraminidase gene-segment packaging while enhancing viral fitness and transmissibility. Proc. Natl Acad. Sci. USA 111, 16854–16859 (2014).
Brooke, C. B. et al. Most influenza A virions fail to express at least one essential viral protein. J. Virol. 87, 3155–3162 (2013).
Sun, J. & Brooke, C. B. Influenza A virus superinfection potential is regulated by viral genomic heterogeneity. mBio 9, e01761-18 (2018).
Fonville, J. M., Marshall, N., Tao, H., Steel, J. & Lowen, A. C. Influenza virus reassortment is enhanced by semi-infectious particles but can be suppressed by defective interfering particles. PLoS Pathog. 11, e1005204 (2015).
Jacobs, N. T. et al. Incomplete influenza A virus genomes occur frequently but are readiliy complemented during localized viral spread. Nat. Commun. 10, 3526 (2019).
Nayak, D. P. Defective interfering influenza viruses. Annu. Rev. Microbiol. 34, 619–644 (1980).
Von Magnus, P. Incomplete forms of influenza virus. Adv. Virus Res. 2, 59–79 (1954).
Brooke, C. B. Biological activities of ‘noninfectious’ influenza A virus particles. Future Virol. 9, 41–51 (2014).
Timm, C., Gupta, A. & Yin, J. Robust kinetics of an RNA virus: transcription rates are set by genome levels. Biotechnol. Bioeng. 112, 1655–1662 (2015).
Boulle, M. et al. HIV cell-to-cell spread results in earlier onset of viral gene expression by multiple infections per cell. PLoS Pathog. 12, e1005964 (2016).
Sanjuán, R. & Thoulouze, M.-I. Why viruses sometimes disperse in groups? Virus Evol. 5, vez014 (2019).
Sigal, A. et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477, 95–98 (2011).
Webster, R. G., Hinshaw, V. S., Bean, W. J. Jr, Turner, B. & Shortridge, K. F. Influenza viruses from avian and porcine sources and their possible role in the origin of human pandemic strains. Dev. Biol. Stand. 39, 461–468 (1977).
Wright, P. F., Neumann, G. & Kawaoka, Y. in Fields Virology Vol. 1 (eds Howley. P. M. & Knipe, D. M.) 1691–1740 (Lippincott-Raven, 2006).
Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T. M. & Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56, 152–179 (1992).
Webster, R. G., Shortridge, K. F. & Kawaoka, Y. Influenza: interspecies transmission and emergence of new pandemics. FEMS Immunol. Med. Microbiol. 18, 275–279 (1997).
Taubenberger, J. K. & Morens, D. M. 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 12, 15–22 (2006).
Viboud, C., Miller, M., Olson, D., Osterholm, M. & Simonsen, L. Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr., RRN1153 (2010).
Marshall, N., Priyamvada, L., Ende, Z., Steel, J. & Lowen, A. C. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog. 9, e1003421 (2013).
Perez, D. R. et al. Role of quail in the interspecies transmission of H9 influenza A viruses: molecular changes on HA that correspond to adaptation from ducks to chickens. J. Virol. 77, 3148–3156 (2003).
Russell, A. B., Trapnell, C. & Bloom, J. D. Extreme heterogeneity of influenza virus infection in single cells. eLife 7, e32303 (2018).
Ramos, I. et al. Innate immune response to influenza virus at single-cell resolution in human epithelial cells revealed paracrine induction of interferon lambda 1. J. Virol. 93, e00559-19 (2019).
Cristobal Vera, J. et al. A common pattern of influenza A virus single cell gene expression heterogeneity governs the innate antiviral response to infection. Preprint at bioRxiv, https://doi.org/10.1101/858373 (2019).
Zheng, G. X. et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 8, 14049 (2017).
Butt, K. M. et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 43, 5760–5767 (2005).
Peiris, M. et al. Human infection with influenza H9N2. Lancet 354, 916–917 (1999).
Guan, Y. et al. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74, 9372–9380 (2000).
Guan, Y., Shortridge, K. F., Krauss, S. & Webster, R. G. Molecular characterization of H9N2 influenza viruses: were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl Acad. Sci. USA 96, 9363–9367 (1999).
Lam, T. T. et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature 502, 241–244 (2013).
Wu, A. et al. Sequential reassortments underlie diverse influenza H7N9 genotypes in China. Cell Host Microbe 4, 446–452 (2013).
Jacobs, N. T. et al. Incomplete influenza A virus genomes occur frequently but are readily complemented during localized viral spread. Nat. Commun. 10, 3526 (2019).
McCrone, J. T. et al. Stochastic processes constrain the within and between host evolution of influenza virus. eLife 7, e35962 (2018).
Valesano, A. L. et al. Influenza B viruses exhibit lower within-host diversity than influenza A viruses in human hosts. J. Virol. 94, e01710-19 (2020).
Jagger, B. W. et al. An overlapping protein-coding region in influenza A virus segment 3 modulates the host response. Science 337, 199–204 (2012).
Te Velthuis, A. J. & Fodor, E. Influenza virus RNA polymerase: insights into the mechanisms of viral RNA synthesis. Nat. Rev. Microbiol. 14, 479–493 (2016).
Dias, A. et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458, 914–918 (2009).
Fan, H. et al. Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature 573, 287–290 (2019).
Gaucherand, L. et al. The influenza A virus endoribonuclease PA-X usurps host mRNA processing machinery to limit host gene expression. Cell Rep. 27, 776–792 (2019).
Khaperskyy, D. A., Schmaling, S., Larkins-Ford, J., McCormick, C. & Gaglia, M. M. Selective degradation of host RNA polymerase II transcripts by influenza A virus PA-X host shutoff protein. PLoS Pathog. 12, e1005427 (2016).
Chao, L., Tran, T. & Matthews, C. Muller’s Ratchet and the advantage of sex in the RNA virus φ6. Evolution 46, 289–299 (1992).
Froissart, R. et al. Co-infection weakens selection against epistatic mutations in RNA viruses. Genetics 168, 9–19 (2004).
Novella, I. S., Reissig, D. D. & Wilke, C. O. Density-dependent selection in vesicular stomatitis virus. J. Virol. 78, 5799–5804 (2004).
Wilke, C. O. & Novella, I. S. Phenotypic mixing and hiding may contribute to memory in viral quasispecies. BMC Microbiol. 3, 11 (2003).
Danzy, S. et al. Mutations to PB2 and NP proteins of an avian influenza virus combine to confer efficient growth in primary human respiratory cells. J. Virol. 88, 13436–13446 (2014).
Hoffmann, E., Neumann, G., Kawaoka, Y., Hobom, G. & Webster, R. G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl Acad. Sci. USA 97, 6108–6113 (2000).
Schwartz, S. L. & Lowen, A. C. Droplet digital PCR: a novel method for detection of influenza virus defective interfering particles. J. Virol. Methods 237, 159–165 (2016).
Perez, D. R., Webby, R. J., Hoffmann, E. & Webster, R. G. Land-based birds as potential disseminators of avian mammalian reassortant influenza A viruses. Avian Dis. 47, 1114–1117 (2003).
Song, H., Nieto, G. R. & Perez, D. R. A new generation of modified live-attenuated avian influenza viruses using a two-strategy combination as potential vaccine candidates. J. Virol. 81, 9238–9248 (2007).
Sorrell, E. M., Wan, H., Araya, Y., Song, H. & Perez, D. R. Minimal molecular constraints for respiratory droplet transmission of an avian–human H9N2 influenza A virus. Proc. Natl Acad. Sci. USA 106, 7565–7570 (2009).
Brown, J. D. et al. Intestinal excretion of a wild bird-origin H3N8 low pathogenic avian influenza virus in mallards (Anas platyrhynchos). J. Wildl. Dis. 48, 991–998 (2012).
Chen, H. et al. Partial and full PCR-based reverse genetics strategy for influenza viruses. PLoS ONE 7, e46378 (2012).
Matlin, K. S., Reggio, H., Helenius, A. & Simons, K. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91, 601–613 (1981).
Yoshimura, A. & Ohnishi, S. Uncoating of influenza virus in endosomes. J. Virol. 51, 497–504 (1984).
Phipps, K. L. et al. Seasonal H3N2 and 2009 pandemic H1N1 influenza A viruses reassort efficiently but produce attenuated progeny. J. Virol. 91, e00830-17 (2017).
Reed, L. J. & Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27, 493–497 (1938).
Campbell, P. J. et al. The M segment of the 2009 pandemic influenza virus confers increased NA activity, filamentous morphology and efficient contact transmissibility to A/Puerto Rico/8/1934-based reassortant viruses. J. Virol. 88, 3802–3814 (2014).
Wittwer, C. T., Reed, G. H., Gundry, C. N., Vandersteen, J. G. & Pryor, R. J. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin. Chem. 49, 853–860 (2003).
Richard, M., Herfst, S., Tao, H., Jacobs, N. T. & Lowen, A. C. Influenza A virus reassortment is limited by anatomical compartmentalization following co-infection via distinct routes. J. Virol. 92, e02063-17 (2017).
Simpson, E. H. Measurement of diversity. Nature 163, 688 (1949).
Hill, M. O. Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–432 (1973).
Jost, L. Entropy and diversity. Oikos 113, 363–375 (2006).
Ward, J. Hierarchical grouping to optimize an objective function. J. Am. Statistical Assoc. 48, 236–244 (1963).
Zhou, B. et al. Single-reaction genomic amplification accelerates sequencing and vaccine production for classical and Swine origin human influenza a viruses. J. Virol. 83, 10309–10313 (2009).
Drayman, N., Kler, S., Ben-nun-Shaul, O. & Oppenheim, A. Rapid method for SV40 titration. J. Virol. Methods 164, 145–147 (2010).
Kawakami, E. et al. Strand-specific real-time RT-PCR for distinguishing influenza vRNA, cRNA, and mRNA. J. Virol. Methods 173, 1–6 (2011).
Pflug, A., Guilligay, D., Reich, S. & Cusack, S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516, 355–360 (2014).
Lukarska, M. et al. Structural basis of an essential interaction between influenza polymerase and Pol II CTD. Nature 541, 117–121 (2017).
Acknowledgements
We thank D. Stallknecht (University of Georgia) for providing the A/mallard/MN/199106/99 (H3N8) biological isolate. We thank J. Shartouny for helpful discussions and the preparation of Extended Data Fig. 7. We thank H. Tao, S. Danzy and G. Geiger for technical assistance. This work was funded in part by the NIH (grant no. R01 AI127799 to A.C.L. and D.R.P.) and NIH/NIAID Centers of Excellence in Influenza Research and Surveillance (CEIRS; grant no. HHSN272201400004C to A.C.L. and G.S.T., and HHSN272201400008C to D.R.P.). Additional funds were provided by the Georgia Research Alliance and the Georgia Poultry Federation (to D.R.P.) and NIH/NIAID Genomic Centers for Infectious Diseases (GCID; grant no. U19 AI110819 to G.S.T.). K.L.P. was supported by T32 grant no. AI106699.
Author information
Authors and Affiliations
Contributions
K.L.P. contributed to the conception of the work, experimental design, data acquisition, and analysis and interpretation of data. K.G., N.T.J. and C.-Y.L. contributed to the experimental design, data acquisition, and analysis and interpretation of data. S.C. contributed to data acquisition. M.M. and M.C.W. contributed to data acquisition and analysis. B.E.P. contributed to data analysis and interpretation. G.S.T. contributed to the conception of the work, and data analysis and interpretation. L.M.F. contributed to data acquisition. D.R.P. contributed to the experimental design, and data analysis and interpretation. A.C.L. contributed to the conception of the work, experimental design, and data analysis and interpretation. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Level of infection achieved in single-cycle growth assays, as determined by flow cytometric detection of HA protein.
Triplicate or duplicate wells of cells were harvested at 24 h post-infection and stained to detect surface expression of HA and HIS epitope tags. Panel a, corresponds to Extended Data Fig. 2A-C and Panel b, corresponds to Extended Data Fig. 2E-F. Lines connect the means of n = 2 or n = 3 replicate samples. c, Flow gating was performed by excluding cell debris and multiplet cells. Quadrant gates were used to quantify each population. Flow cytometry results are representative of those obtained in three independent experiments.
Extended Data Fig. 2 Increasing the m.o.i. increases viral productivity at sub-saturating but not saturating m.o.i.
Data relate to Fig. 2. MDCK or DF-1 cells were infected under single-cycle conditions at a range of MOIs. Low MOI range is shown in panels a–d, and high MOI range is shown in panels e, f. As shown in Extended Data Fig. 1, MOIs < 1 PFU/cell were found to be sub-saturating. Viral titres observed at the indicated MOIs are plotted against time post-infection for GFHK99 virus in MDCK cells (a), GFHK99 virus in DF-1 cells (b), MaMN99 virus in MDCK cells (c), NL09 virus in MDCK cells (d), GFHK99 virus in MDCK cells (e), and GFHK99 virus in DF-1 cells (f). Lines connect the mean values for technical replicates sampled at each time point.
Extended Data Fig. 3 Introduction of the PA gene segment from GFHK99 virus into MaMN99 virus confers increased dependence on multiple infection for vRNA synthesis.
Cells were coinfected with WT virus and increasing doses of VAR virus. WT virus MOI was 0.005 PFU per cell. The fold change in WT vRNA copy number, relative to that detected in the absence of VAR virus, is plotted for MaMN99 virus a, and MaMN99-GFHK99-PA virus b. Bars represent the mean of n = 3 replicate cell cultures per condition. Data shown in panel (a) are also shown in Fig. 3. MaMN99 virus was tested in MDCK and DE cells; MaMN99 GFHK99-PA virus was tested in MDCK and DF-1 cells.
Extended Data Fig. 4 A high m.o.i. is needed for robust GFHK99 polymerase activity in MDCK cells.
MDCK or DF-1 cells were infected with GFHK99 or MaMN99 virus at low (0.5 RNA copies per cell) or high (3 HA-expressing units per cell) MOI. NS segment vRNA and mRNA was quantified at the indicated time points a–f. The average fold change from initial (t = 0) to peak RNA copy number is plotted for low MOI infections g, and high MOI infections h. Mean and standard deviation are plotted for n = 3 replicate cell cultures sampled sequentially. Significance was assessed by multiple unpaired, two-sided t-tests with correction for multiple comparisons using the Holm-Sidak method, with alpha = 5.0%. Each row was analysed individually, without assuming a consistent SD.
Extended Data Fig. 5 Preliminary analysis of single-cell mRNA sequencing data to exclude cells with viral mRNA that are likely uninfected.
a, Within each infection, cells in which viral RNA was detected were rank ordered by the proportion of their transcriptome that comprised viral RNA (% viral RNA), and the relative gain in % viral RNA from one cell to the next was plotted against the proportion of viral RNA in each cell. Local regression was performed separately for each infection, and the first local minimum of the resulting functions (indicated by dashed lines) indicated the point at which the marginal gain in % viral RNA was more consistent and less sensitive to the % viral RNA of the prior cell. Cells with % viral RNA values below this threshold were deemed falsely positive and considered uninfected for the analyses shown in Fig. 5 and Extended Data Fig. 6. Facets indicate individual infections, with lines coloured by cell type (DF-1 = pink, MDCK = blue). b, The same analysis in panel A) was applied to the data from the second experiment, in which cells were co-inoculated with a 1:1 mixture of WT and mVAR1 viruses, as well as mVAR2 virus at an MOI of 0.1 PFU per cell in DF-1 cells, or 1.0 PFU per cell in MDCK cells. Only cells containing all eight mVAR2 segments were analysed in this manner.
Extended Data Fig. 6 Validation of single-cell mRNA sequencing data.
a, b, e, In the first single-cell sequencing experiment, DF-1 or MDCK cells were infected with GFHK99 WT virus at four different MOIs (0.07, 0.2, 0.6, 1.8 NP units per cell), and the transcriptomes of individual cells were sequenced using the 10x Genomics Chromium platform (n = 1,228 DF-1 cells, 645 MDCK cells, 1 sequencing replicate per infection condition). a, The total number of cells sequenced, infected, and containing PB2, PB1, PA, and NP segments are represented by the cumulative heights of the grey, light yellow, and dark yellow bars, respectively. Cells that were excluded by the analysis shown in Extended Data Fig. 5 are contained within the grey bar. b, Each violin plot shows the full distribution of log10-transformed viral mRNA abundance, for all eight viral transcripts combined, in individual infected cells. Vertical lines represent the median of each distribution. The data are stratified by cell type (MDCK cells in blue, DF-1 cells in pink), MOI, and the presence of polymerase complex (light shading = cells missing PB2, PB1, PA, or NP; dark shading = cells in which PB2, PB1, PA and NP are all detected). The absence of a dark shaded distribution for MDCK cells at the lowest MOI is due to the absence of any cells in which all four of these segments were detected. c, d, e, In the second single-cell sequencing experiment, DF-1 or MDCK cells were infected with GFHK99 WT and mVAR1 viruses at total MOIs of 0.07, 0.2, 0.6, 1.8 NP units per cell, and simultaneously with a constant dose of mVAR2 virus (n = 462 DF-1 cells, 671 MDCK cells, 1 sequencing replicate per infection condition). c, The total number of cells sequenced, containing all eight mVAR2 genome segments, and infected with either WT or mVAR1 virus are represented by the cumulative heights of the grey, light orange, and dark orange bars, respectively. As in panel (A), cells that were deemed falsely positive are contained within the grey bar. d, Distributions of viral UMIs per cell are shown separately for WT (bottom of each cell-MOI pair) and mVAR1 (top of each cell-MOI pair). Vertical lines represent the median of each distribution. As expected, no significant difference was detected between WT and mVAR1 transcript levels (p = 0.061, linear mixed effects model). e, The distributions of total UMIs detected per cell are shown for each cell type, MOI, and infection type, from both experiments. Vertical lines represent the median of each distribution.
Extended Data Fig. 7 Alignment of MaMN99 and GFHK99 virus PA and PA-X amino acid sequences.
Sequences and functional domains of the PA protein are displayed in panel a, and those of the PA-X protein are shown in panel b. N-ter = the N-terminal endonuclease domain;69 C-ter = C-terminal domain;69 X-ORF = the 61 amino acid region of PA-X encoded in frame 2 of the PA gene;37 Active site = the active site of the endonuclease;39 Dim. Loop = dimerization loop important for formation of polymerase dimers;40 Site 1 and Site 2 = sites mediating the interaction of PA with cellular Pol II C-terminal domain70.
Extended Data Fig. 8 Quantification of defective interfering RNA content in virus stocks.
Defective interfering RNA content for a, PB2, b, PB1 and c, PA segments was determined by ddPCR using primer pairs targeting terminal and internal portions of each polymerase gene segment to determine their absolute copy number and produce a ratio of terminal:internal copies. All virus stocks used in this study contained low DI content (terminal:internal ratio less than or equal to 2). A DI-rich control virus, Pan99wt P3 (A/Panama/2007/99 [H3N2]), is included for comparison. This virus stock was passaged three times in MDCK cells at high MOI. For the MaMN99-GFHK99 chimeric viruses, the segments derived from GFHK99 virus are listed in place of the full strain names.
Extended Data Fig. 9 Example gating for flow cytometry to evaluate HA positive cell numbers.
Plots shown were generated in the course of experiments reported in Fig. 1 and are representative of results obtained in at least three independent experiments. Following staining for cell-surface HA protein: 1) A population of cells was selected by gating out cell debris by SSC-A vs FSC-A. 2) Multiplets were excluded by gating for single cells in SSC-H vs SSC-W. 3) Populations of infected cells were gated based on expression of the appropriate epitope tag.
Extended Data Fig. 10 Titration of virus stocks for HA-expressing units and NP-expressing units by flow cytometry.
a, The doses to be used in RNA kinetics studies shown in Extended Data Fig. 4 were determined via flow titration of HA-expressing units in the relevant cell lines. GFHK99 and MaMN99 virus mixtures were titrated in MDCK and DF-1 cells to calculate HA-‘expressing units/mL for each virus-cell combination. Serial dilutions of virus were used to infect cells under synchronized, single cycle conditions. Cells were harvested at 24 h post infection and stained for epitope tags. Data within the linear range were used to calculate the viral titre. b, GFHK99 viruses used in mRNA sequencing experiments shown in Fig. 5 were titered in DF-1 cells. DF-1 cells are more permissive to infection and thus give more sensitive detection of infectious virus compared to MDCK cells. As the virus strains used did not contain epitope tags, virus detection was accomplished through cell permeabilization and detection of the viral NP protein. Data within the linear range were used to calculate viral titres. Representative flow plots show gates used following exclusion of cell debris and doublets.
Supplementary information
Supplementary information
Supplementary Tables 1–4.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Phipps, K.L., Ganti, K., Jacobs, N.T. et al. Collective interactions augment influenza A virus replication in a host-dependent manner. Nat Microbiol 5, 1158–1169 (2020). https://doi.org/10.1038/s41564-020-0749-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0749-2
This article is cited by
-
Influenza A virus reassortment in mammals gives rise to genetically distinct within-host subpopulations
Nature Communications (2022)
-
Influenza virus hedges its bets
Nature Reviews Microbiology (2020)