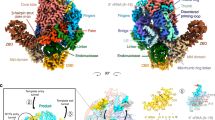
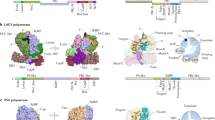
Abstract
Segmented negative-sense RNA viruses (sNSRVs) encode a single-polypeptide polymerase (L protein) or a heterotrimeric polymerase complex to cannibalize host messenger RNA cap structures serving as primers of transcription, and catalyse RNA synthesis. Here, we report the full-length structure of the severe fever with thrombocytopaenia syndrome virus (SFTSV) L protein, as determined by cryogenic electron microscopy at 3.4 Å, leading to an atomic model harbouring three functional parts (an endonuclease, an RNA-dependent RNA polymerase and a cap-binding domain) and two structural domains (an arm domain with a blocker motif and a carboxy-terminal lariat domain). The SFTSV L protein has a compact architecture in which its cap-binding pocket is surprisingly occupied by an Arg finger of the blocker motif, and the endonuclease active centre faces back towards the cap-binding pocket, suggesting that domain rearrangements are necessary to acquire the pre-initiation state of the active site. Our results provide insight into the complete architecture of sNSRV-encoded L protein and further the understanding of sNSRV transcription initiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41564-021-00906-y
References
Morin, B., Kranzusch, P. J., Rahmeh, A. A. & Whelan, S. P. The polymerase of negative-stranded RNA viruses. Curr. Opin. Virol. 3, 103–110 (2013).
Li, J., Rahmeh, A., Morelli, M. & Whelan, S. P. A conserved motif in region V of the large polymerase proteins of nonsegmented negative-sense RNA viruses that is essential for mRNA capping. J. Virol. 82, 775–784 (2008).
Ogino, T. & Banerjee, A. K. Unconventional mechanism of mRNA capping by the RNA-dependent RNA polymerase of vesicular stomatitis virus. Mol. Cell 25, 85–97 (2007).
Li, J., Wang, J. T. & Whelan, S. P. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc. Natl Acad. Sci. USA 103, 8493–8498 (2006).
Morin, B. et al. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Pathog. 6, e1001038 (2010).
Gogrefe, N., Reindl, S., Gunther, S. & Rosenthal, M. Structure of a functional cap-binding domain in Rift Valley fever virus L protein. PLoS Pathog. 15, e1007829 (2019).
Yuan, P. et al. Crystal structure of an avian influenza polymerase PAN reveals an endonuclease active site. Nature 458, 909–913 (2009).
Dias, A. et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458, 914–918 (2009).
Abudurexiti, A. et al. Taxonomy of the order Bunyavirales: update 2019. Arch. Virol. 164, 1949–1965 (2019).
Guu, T. S., Zheng, W. & Tao, Y. J. Bunyavirus: structure and replication. Adv. Exp. Med. Biol. 726, 245–266 (2012).
Fodor, E. The RNA polymerase of influenza A virus: mechanisms of viral transcription and replication. Acta Virol. 57, 113–122 (2013).
Liang, B. et al. Structure of the L protein of vesicular stomatitis virus from electron cryomicroscopy. Cell 162, 314–327 (2015).
Gilman, M. S. A. et al. Structure of the respiratory syncytial virus polymerase complex. Cell 179, 193–204 (2019).
Pan, J. et al. Structure of the human metapneumovirus polymerase phosphoprotein complex. Nature 577, 275–279 (2020).
Pflug, A., Guilligay, D., Reich, S. & Cusack, S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 516, 355–360 (2014).
Reich, S. et al. Structural insight into cap-snatching and RNA synthesis by influenza polymerase. Nature 516, 361–366 (2014).
Chang, S. et al. Cryo-EM structure of influenza virus RNA polymerase complex at 4.3 A resolution. Mol. Cell 57, 925–935 (2015).
Fan, H. et al. Structures of influenza A virus RNA polymerase offer insight into viral genome replication. Nature 573, 287–290 (2019).
Hengrung, N. et al. Crystal structure of the RNA-dependent RNA polymerase from influenza C virus. Nature 527, 114–117 (2015).
Peng, Q. et al. Structural insight into RNA synthesis by influenza D polymerase. Nat. Microbiol. 4, 1750–1759 (2019).
Gerlach, P., Malet, H., Cusack, S. & Reguera, J. Structural insights into bunyavirus replication and its regulation by the vRNA promoter. Cell 161, 1267–1279 (2015).
Yu, X. J. et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 364, 1523–1532 (2011).
Jiao, L. et al. Structure of severe fever with thrombocytopenia syndrome virus nucleocapsid protein in complex with suramin reveals therapeutic potentials. J. Virol. 87, 6829–6839 (2013).
Zhou, H., Sun, Y., Guo, Y. & Lou, Z. Structural perspective on the formation of ribonucleoprotein complex in negative-sense single-stranded RNA viruses. Trends Microbiol. 21, 475–484 (2013).
Zhou, H. et al. The nucleoprotein of severe fever with thrombocytopenia syndrome virus processes an oligomeric ring to facilitate RNA encapsidation. Protein Cell 4, 445–455 (2013).
Halldorsson, S. et al. Structure of a phleboviral envelope glycoprotein reveals a consolidated model of membrane fusion. Proc. Natl Acad. Sci. USA 113, 7154–7159 (2016).
Wu, Y. et al. Structures of phlebovirus glycoprotein Gn and identification of a neutralizing antibody epitope. Proc. Natl Acad. Sci. USA 114, E7564–E7573 (2017).
2018 Annual Review of Diseases Prioritized Under the Research and Development Blueprint (World Health Organization, 2018); http://www.who.int/emergencies/diseases/2018prioritization-report.pdf
Guilligay, D. et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat. Struct. Mol. Biol. 15, 500–506 (2008).
Thierry, E. et al. Influenza polymerase can adopt an alternative configuration involving a radical repacking of PB2 domains. Mol. Cell 61, 125–137 (2016).
Knizewski, L., Kinch, L. N., Grishin, N. V., Rychlewski, L. & Ginalski, K. Realm of PD-(D/E)XK nuclease superfamily revisited: detection of novel families with modified transitive meta profile searches. BMC Struct. Biol. 7, 40 (2007).
Reguera, J., Weber, F. & Cusack, S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 6, e1001101 (2010).
Wallat, G. D. et al. High-resolution structure of the N-terminal endonuclease domain of the Lassa virus L polymerase in complex with magnesium ions. PLoS ONE 9, e87577 (2014).
Tao, Y., Farsetta, D. L., Nibert, M. L. & Harrison, S. C. RNA synthesis in a cage—structural studies of reovirus polymerase λ3. Cell 111, 733–745 (2002).
Gong, P. & Peersen, O. B. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc. Natl Acad. Sci. USA 107, 22505–22510 (2010).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Hu, M. et al. A particle-filter framework for robust cryo-EM 3D reconstruction. Nat. Methods 15, 1083–1089 (2018).
Tan, Y. Z. et al. Addressing preferred specimen orientation in single-particle cryo-EM through tilting. Nat. Methods 14, 793–796 (2017).
Naydenova, K. & Russo, C. J. Measuring the effects of particle orientation to improve the efficiency of electron cryomicroscopy. Nat. Commun. 8, 629 (2017).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Jones, R. et al. Structure and function of the Toscana virus cap-snatching endonuclease. Nucleic Acids Res. 47, 10914–10930 (2019).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Sehnal, D. et al. MOLE 2.0: advanced approach for analysis of biomacromolecular channels. J. Cheminformatics 5, 39 (2013).
Acknowledgements
We thank the Computing and Cryo-EM platforms of the Tsinghua University Branch of the National Center for Protein Sciences (Beijing) for cryo-EM sample preparation and screening. We acknowledge computational support from Beijing Computational Science Research Center (CSRC) and Beijing Three Axis Space Technology Co. Ltd. This work was supported by the National Program on Key Research Project of China (grant nos. 2020YFA0707500, 2017YFC0840300, 2018YFA0507200 and 2018YFE0200402), National Natural Science Foundation of China (31971126, 31770309, 81520108019 and 31670731) and Nature Science Foundation of Hubei Province (2019CFB790).
Author information
Authors and Affiliations
Contributions
Z.L., Z.R. and F.D. conceived of the project and designed the experiments. Panpan Wang, L.L., L.Y., Y.H., S.S. and Y.G. expressed and purified the proteins. Panpan Wang, L.L. and A.L. prepared the cryo-EM sample. Panpan Wang, A.L., M.H., H.L., C.L. and Peiyi Wang collected and processed the cryo-EM data and built the model. Panpan Wang and Y.L. performed the surface plasmon resonance experiments. Z.L. wrote the manuscript. All authors discussed the experiments and read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Cryo-EM reconstruction.
a, Raw image of SFTSV-L particles in vitreous ice recorded at defocus values of -1.5 to -2.5 μm. Scale bar, 100 nm. b, Power spectrum of the image shown in (a), with plot of the rotationally averaged intensity versus resolution. White circle indicates the spatial frequency corresponding to 3.0 Å resolution. c, Representative class averages. The edge of each square is 238 Å. d, Overview of SFTSV-L reconstruction is shown in the left panel. The close-up view of a representative region in the RdRp region is shown in the right panel. Density is shown as gray mesh; the polypeptide of the refined model is displayed as colored sticks (carbons, green; nitrogen, blue; oxygen, red). e, Fourier shell correlation (FSC) of the final 3D reconstruction following gold standard refinement. FSC curves are plotted before (red) and after (green) masking in addition to post-correction (blue), accounting for the effect of the mask using phase randomization. f, Local resolution estimation was performed with Relion 2.11. g, The 3DFSC sphericity was analyzed with 3DFSC2. h, The data processing scheme used to obtain the final map.
Extended Data Fig. 2 Secondary structure diagram of SFTSV-L.
Secondary structure elements along the SFTSV-L sequence are shown as cylinders and arrows for ɑ-helices and β-strands, respectively. Domains are colored as in Fig. 1.
Extended Data Fig. 3 The lariat domain.
a-c The lariat domain is represented as a cartoon diagram in red covered by cryo-EM density in three views. Other domains of SFTSV-L are covered by molecular surface with the same color as in Fig. 1. d, A close-up view of the lariat domain covered by the cryo-EM density. Residues for tracing the direction of the polypeptide are indicated by dots with labels.
Extended Data Fig. 4 The endoN domain.
a, The contacts between SFTSV-L endoN domain and other domains. The structure of SFTSV-L is displayed with the same color scheme in Fig. 1. The endoN domain is covered by a molecular surface, and other domains are shown as cartoon diagrams. The back view and the side view of the endoN domain are shown in the right panels. The interdomain interfaces on the endoN domain are indicated by the same color as each of the interacting domains, including the RdRp core, the PB2-N-like domain, the arm domain and the blocker motif, the CBD domain and the lariat domain. b, The orientation of the endoN domains in SFTSV-L and LACV-L. The structures of SFTSV-L and LACV-L 1750 are aligned according to their RdRp domains. The endoN domains of SFTSV-L and LACV-L are covered by molecular surface in forest green and smudge green, respectively. The active residues of each endoN domain are highlighted in orange. The polypeptide of other domains in SFTSV-L was represented as cartoon diagram. The cap-binding pocket in the CBD domain is highlighted by a red dotted circle. The distance from the cap-binding pocket to the position of the active center of LACV-L endoN domain is approximately 80 Å.
Extended Data Fig. 5 Orientation of the CBD domain and the blocker motif.
This figure is related to Fig. 2. The structures of SFTSV-L a, FluA-vRNA PA-PB1-PB2 complex b, FluB1-vRNA PA-PB1-PB2 complex c, FluB-cRNA PA-PB1-PB2 complex d, and apo FluC PA-PB1-PB2 complex e, are aligned according to their CBD domains and are shown in the same orientation in the upper panels. Because the CBD domains from these structures are located different site from their own RdRp cores, other domains of these structures are in significantly distinct positions. In the lower panels, the CBD domain and its interacting fragments in these structures are shown in detail. The CBD domains are covered by molecular surface, and the other fragments are shown as cartoon diagrams. The residues constituting the cap-binding pockets are highlighted in red and the PB2 cap-627 linker3 is in blue. Note, the orientation of the detailed illustrations for FluB1 (c), FluB (d) and FluC (e) has a ~90° clockwise rotation for clear presentation. The residues occupy the cap-binding pockets of SFTSV-L and apo FluC PA-PB1-PB2 complex are displayed as spheres.
Extended Data Fig. 6 Comparison of subdomains in the RdRp region.
The structures of the RdRp region from SFTSV-L, LACV-L and IAV PA-PB1-PB2 complex are in the same orientation and color scheme as in Fig. 4b. The PA-C-like (or PA-C) domain, the RdRp core, and the PB2-N-like (or PB2-N) domain are shown separately in the right, middle and left panels.
Extended Data Fig. 7 Comparison of the priming loop.
a-d. The subdomains of the RdRp core and the PA-C-like domain of SFTSV-L (a), LACV-L (PDB code: 5AMR) (c) and FluA PA-PB1-PB2 (PDB code: 4WSB) (d) are covered by a molecular surface with the same color scheme as that in Fig. 4b. The priming loops in three structures are shown as white cartoons. The priming loop in SFTSV-L is enlarged and is covered with cryo-EM density in (b). The RdRp catalytic centers of the three polymerases are highlighted by the white circles. e, Density of motif F. The RdRp motifs A, B, D, F, H of SFTSV-L are shown as cartoon diagram with the same color scheme as that of Fig. 5a. Motif F is covered by the cryo-EM density.
Extended Data Fig. 8 The active site of the RdRp domain.
a, The details of the RdRp motifs of SFTSV-L. This figure is generally the same as Fig. 5a, with one exception that the cation, assumed to be a magnesium ion, is shown as a red sphere. In contrast, two catalytic manganese ions from the polio virus elongation complex structure4 are shown as black spheres. b, The bound cation and surrounding residues are covered by cryo-EM densities. The bonds formed between SFTSV-L polypeptide and the cation are labeled with distance. c, The electrostatic surface potential of SFTSV-L RdRp tunnels. The positive surface is colored blue, the negative surface, red, with limits ±70 kbT/ec. The regions belong to the NTP entry, template entry, template exit and product exit tunnles are indicated out by dashed lines. d, The entrance of NTP entry tunnel, and e, the entrance of template entry tunnel. Colors are as in Fig. 4. Superposition of LACV-L 1750 (5AMR) structure shows the position of the 5' vRNA (gray cartoon) in (b). The approximate positions of NTP and template entry tunnels are bordered by dashed lines.
Extended Data Fig. 9 Impact of the endoN domain on the product exit tunnel.
This figure is related to Fig. 5b. a, Same as Fig. 5b, the tunnels for template entry, NTP entry, product exit, and template exit of SFVSV-L are indicated with violet, salmon red, orange and green labels in (a). b, A model without the endoN domain and residues 206-217 in the linker region was generated, and its tunnels were calculated and shown as the same color scheme in (a). The bottom views of the product exit tunnel in SFTSV-L c, or the model without the endoN domain and linker residues d, is shown.
Extended Data Fig. 10 Proposed models for transcription initiation and co-factor binding.
a, A proposed speculative model of SFTSV transcription initiation. SFTSV-L is schematically represented, with the cap-binding pocket (red hollow stars) in the CBD domain, the RdRp catalytic center (deep red dots) and the active center of the endoN domain (orange stars) as marked. (1) During the early event of the cap-snatching process, the lariat domain loses its interaction with the RdRp domain to allow the L protein to transform into a fully open state. Meanwhile, the endoN domain is pulled out from the central position of the SFTSV-L, and the arm domain shifts to induce the removal of the Arg-finger in the block motif from the cap-binding pocket of the CBD domain. (2) After the host mRNA is captured, the endoN domain employs an ~180° rotation along its long axis, thus causing its active center to face the nucleotide chain of host mRNA, and cleaves it by using two cations as cofactors chelated by the conserved H...PD...D/E...K motif. If the structures of LACV-L 1750 and SFTSV-L are aligned using their RdRp cores as the reference, the cap-binding pocket in the SFTSV-L CBD domain will have a distance of ~80 Å relative to the position of the LACV-L endoN domain active center (Supplementary Fig. 5). We reason the lack of C-terminal parts in the structure of LACV-L 1750 abolishes the extensive interdomain interactions to maintain the compact structure and allow its endoN domain to be in a fully open state. (3) Then, the CBD anchors the snatched cap structure with additional nucleotides and transfers the 3' end nucleotide chain to the RdRp catalytic cavity. (4) The transcription of virus mRNA is thus elongated with the guidance by vRNA as the template, and the products will be released from the product exit tunnel. In this stage, the endoN domain is likely to stay out of the center of the L protein, allowing the product exit tunnel to be in a fully open state. Meanwhile, the lariat motif will go back to its initial state. b, The structures of SFTSV-L and RSV-L-P complex5 are aligned by their RdRp core and shown in the same orientation. The colors for SFTSV-L domains are the same as those in Fig. 1. The RdRp and the PRNTase domains of RSV-L are shown in pale cyan and cyan, respectively. The P proteins bound to RSV-L is shown as cartoon diagram, and its structurally equivalent position in SFTSV-L is framed out.
Supplementary information
Supplementary Information
Supplementary Fig. 1 and Table 1.
Rights and permissions
About this article
Cite this article
Wang, P., Liu, L., Liu, A. et al. Structure of severe fever with thrombocytopenia syndrome virus L protein elucidates the mechanisms of viral transcription initiation. Nat Microbiol 5, 864–871 (2020). https://doi.org/10.1038/s41564-020-0712-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0712-2
This article is cited by
-
Structural characterization of the oligomerization of full-length Hantaan virus polymerase into symmetric dimers and hexamers
Nature Communications (2024)
-
Structural basis for dimerization of a paramyxovirus polymerase complex
Nature Communications (2024)
-
Structural and functional analysis of the minimal orthomyxovirus-like polymerase of Tilapia Lake Virus from the highly diverged Amnoonviridae family
Nature Communications (2023)
-
Molecular mechanism of de novo replication by the Ebola virus polymerase
Nature (2023)
-
An intermediate state allows influenza polymerase to switch smoothly between transcription and replication cycles
Nature Structural & Molecular Biology (2023)