Abstract
The arms race among microorganisms is a key driver in the evolution of not only the weapons but also defence mechanisms. Many Gram-negative bacteria use the type six secretion system (T6SS) to deliver toxic effectors directly into neighbouring cells. Defence against effectors requires cognate immunity proteins. However, here we show immunity-independent protection mediated by envelope stress responses in Escherichia coli and Vibrio cholerae against a V. cholerae T6SS effector, TseH. We demonstrate that TseH is a PAAR-dependent species-specific effector highly potent against Aeromonas species but not against its V. cholerae immunity mutant or E. coli. A structural analysis reveals TseH is probably a NlpC/P60-family cysteine endopeptidase. We determine that two envelope stress-response pathways, Rcs and BaeSR, protect E. coli from TseH toxicity by mechanisms including capsule synthesis. The two-component system WigKR (VxrAB) is critical for protecting V. cholerae from its own T6SS despite expressing immunity genes. WigR also regulates T6SS expression, suggesting a dual role in attack and defence. This deepens our understanding of how bacteria survive T6SS attacks and suggests that defence against the T6SS represents a major selective pressure driving the evolution of species-specific effectors and protective mechanisms mediated by envelope stress responses and capsule synthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lewin, R. Finches show competition in ecology. Science 219, 1411–1412 (1983).
Pukatzki, S. et al. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl Acad. Sci. USA 103, 1528–1533 (2006).
Hood, R. D. et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7, 25–37 (2010).
Altindis, E., Dong, T., Catalano, C. & Mekalanos, J. Secretome analysis of Vibrio cholerae type VI secretion system reveals a new effector-immunity pair. mBio 6, e00075-15 (2015).
Dong, T. G., Ho, B. T., Yoder-Himes, D. R. & Mekalanos, J. J. Identification of T6SS-dependent effector and immunity proteins by Tn-seq in Vibrio cholerae. Proc. Natl Acad. Sci. USA 110, 2623–2628 (2013).
Miyata, S. T., Kitaoka, M., Brooks, T. M., McAuley, S. B. & Pukatzki, S. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect. Immun. 79, 2941–2949 (2011).
Russell, A. B. et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475, 343–347 (2011).
Miyata, S. T., Unterweger, D., Rudko, S. P. & Pukatzki, S. Dual expression profile of type VI secretion system immunity genes protects pandemic Vibrio cholerae. PLoS Pathog. 9, e1003752 (2013).
Logan, S. L. et al. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc. Natl Acad. Sci. USA 115, E3779–E3787 (2018).
Fast, D., Kostiuk, B., Foley, E. & Pukatzki, S. Commensal pathogen competition impacts host viability. Proc. Natl Acad. Sci. USA 115, 7099–7104 (2018).
Fu, Y., Ho, B. T. & Mekalanos, J. J. Tracking Vibrio cholerae cell-cell interactions during infection reveals bacterial population dynamics within intestinal microenvironments. Cell Host Microbe 23, 274–281 (2018).
Speare, L. et al. Bacterial symbionts use a type VI secretion system to eliminate competitors in their natural host. Proc. Natl Acad. Sci. USA 115, E8528–E8537 (2018).
Bingle, L. E., Bailey, C. M. & Pallen, M. J. Type VI secretion: a beginner’s guide. Curr. Opin. Microbiol. 11, 3–8 (2008).
Liang, X. et al. An onboard checking mechanism ensures effector delivery of the type VI secretion system in Vibrio cholerae. Proc. Natl Acad. Sci. USA 116, 23292–23298 (2019).
Senderovich, Y., Gershtein, Y., Halewa, E. & Halpern, M. Vibrio cholerae and Aeromonas: do they share a mutual host?. ISME J. 2, 276–283 (2008).
Holm, L. Benchmarking fold detection by DaliLite v.5. Bioinformatics 35, 5326–5327 (2019).
Xu, Q. et al. Structural analysis of papain-like NlpC/P60 superfamily enzymes with a circularly permuted topology reveals potential lipid binding sites. PLoS ONE 6, e22013 (2011).
Chou, S. et al. Structure of a peptidoglycan amidase effector targeted to Gram-negative bacteria by the type VI secretion system. Cell Rep. 1, 656–664 (2012).
Zhang, Y. & Skolnick, J. TM-align: a protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 33, 2302–2309 (2005).
Anantharaman, V. & Aravind, L. Evolutionary history, structural features and biochemical diversity of the NlpC/P60 superfamily of enzymes. Genome Biol. 4, R11 (2003).
Xu, Q. et al. Structures of a bifunctional cell wall hydrolase CwlT containing a novel bacterial lysozyme and an NlpC/P60 DL-endopeptidase. J. Mol. Biol. 426, 169–184 (2014).
Xu, Q. et al. Structural basis of murein peptide specificity of a γ-d-glutamyl-l-diamino acid endopeptidase. Structure 17, 303–313 (2009).
Laubacher, M. E. & Ades, S. E. The Rcs phosphorelay is a cell envelope stress response activated by peptidoglycan stress and contributes to intrinsic antibiotic resistance. J. Bacteriol. 190, 2065–2074 (2008).
Raffa, R. G. & Raivio, T. L. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45, 1599–1611 (2002).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006 (2006).
Bury-Moné, S. et al. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 5, e1000651 (2009).
Boulanger, A. et al. Multistress regulation in Escherichia coli: expression of osmB involves two independent promoters responding either to σS or to the RcsCDB His-Asp phosphorelay. J. Bacteriol. 187, 3282–3286 (2005).
Francez-Charlot, A., Castanié-Cornet, M.-P., Gutierrez, C. & Cam, K. Osmotic regulation of the Escherichia coli bdm (biofilm-dependent modulation) gene by the RcsCDB His-Asp phosphorelay. J. Bacteriol. 187, 3873–3877 (2005).
Ranjit, D. K. & Young, K. D. Colanic acid intermediates prevent de novo shape recovery of Escherichia coli spheroplasts, calling into question biological roles previously attributed to colanic acid. J. Bacteriol. 198, 1230–1240 (2016).
Gottesman, S., Trisler, P. & Torres-Cabassa, A. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol. 162, 1111–1119 (1985).
Stout, V., Torres-Cabassa, A., Maurizi, M. R., Gutnick, D. & Gottesman, S. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol. 173, 1738–1747 (1991).
Nishino, K., Honda, T. & Yamaguchi, A. Genome-wide analyses of Escherichia coli gene expression responsive to the BaeSR two-component regulatory system. J. Bacteriol. 187, 1763–1772 (2005).
Strauch, K. L. & Beckwith, J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl Acad. Sci. USA 85, 1576–1580 (1988).
Hagenmaier, S., Stierhof, Y. D. & Henning, U. A new periplasmic protein of Escherichia coli which is synthesized in spheroplasts but not in intact cells. J. Bacteriol. 179, 2073–2076 (1997).
Quan, S. et al. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nat. Struct. Mol. Biol. 18, 262–269 (2011).
Weaver, A. I. et al. Genetic determinants of penicillin tolerance in Vibrio cholerae. Antimicrob. Agents Chemother. 62, e01326-18 (2018).
Dörr, T. et al. A cell wall damage response mediated by a sensor kinase/response regulator pair enables beta-lactam tolerance. Proc. Natl Acad. Sci. USA 113, 404–409 (2016).
Teschler, J. K., Cheng, A. T. & Yildiz, F. H. The two-component signal transduction system VxrAB positively regulates Vibrio cholerae biofilm formation. J. Bacteriol. 199, e00139-17 (2017).
Toska, J., Ho, B. T. & Mekalanos, J. J. Exopolysaccharide protects Vibrio cholerae from exogenous attacks by the type 6 secretion system. Proc. Natl Acad. Sci. USA 115, 7997–8002 (2018).
Fong, J. C. N., Syed, K. A., Klose, K. E. & Yildiz, F. H. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology 156, 2757–2769 (2010).
Wong, M. et al. Microbial herd protection mediated by antagonistic interaction in polymicrobial communities. Appl. Environ. Microbiol. 82, 6881–6888 (2016).
Cheng, A. T., Ottemann, K. M. & Yildiz, F. H. Vibrio cholerae response regulator VxrB controls colonization and regulates the type vi secretion system. PLoS Pathog. 11, e1004933 (2015).
Troselj, V., Treuner-Lange, A., Søgaard-Andersen, L. & Wall, D. Physiological heterogeneity triggers sibling conflict mediated by the type VI secretion system in an aggregative multicellular bacterium. mBio 9, e01645-17 (2018).
Lazzaro, M., Feldman, M. F. & García Véscovi, E. A transcriptional regulatory mechanism finely tunes the firing of type VI secretion system in response to bacterial enemies. mBio 8, e00559-17 (2017).
LaCourse, K. D. et al. Conditional toxicity and synergy drive diversity among antibacterial effectors. Nat. Microbiol. 3, 440–446 (2018).
Borenstein, D. B., Ringel, P., Basler, M. & Wingreen, N. S. Established microbial colonies can survive type VI secretion assault. PLoS Comput. Biol. 11, e1004520 (2015).
Dong, T. G. et al. Generation of reactive oxygen species by lethal attacks from competing microbes. Proc. Natl Acad. Sci. USA 112, 2181–2186 (2015).
MacIntyre, D. L., Miyata, S. T., Kitaoka, M. & Pukatzki, S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl Acad. Sci. USA 107, 19520–19524 (2010).
Stietz, M. S., Liang, X., Wong, M., Hersch, S. & Dong, T. G. Double tubular contractile structure of the type VI secretion system displays striking flexibility and elasticity. J. Bacteriol. 202, e00425-19 (2019).
Dong, A. et al. In situ proteolysis for protein crystallization and structure determination. Nat. Methods 4, 1019–1021 (2007).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D 62, 859–866 (2006).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Vonrhein, C., Blanc, E., Roversi, P. & Bricogne, G. in Macromolecular Crystallography Protocols. Structure Determination Vol. 2 (ed. Doublié, S.) 215–230 (Humana Press, 2007).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Burkinshaw, B. J. et al. A type VI secretion system effector delivery mechanism dependent on PAAR and a chaperone–co-chaperone complex. Nat. Microbiol. 3, 632–640 (2018).
Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 47, D590–D595 (2019).
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and Canadian Natural Sciences and Engineering Research Council (NSERC) to T.G.D., and in part by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract nos HHSN272201200026C and HHSN272201700060C to A.S. T.G.D. is also supported by a Government of Canada Research Chair award and a Canadian Foundation for Innovation grant (CFI-JELF). A.P. was funded by the Markin Undergraduate Student Research Program in Health and Wellness. L.L. is supported by an Alberta Innovates Health Solutions (AIHS) graduate student scholarship and K.M. by an Alberta Queen Elizabeth II Graduate Scholarship. B.B. was supported by AIHS and National Sciences and Engineering Research Council of Canada (NSERC) postdoctoral fellowships. S.J.H. holds a CIHR postdoctoral fellowship. We thank Z. Eltsova for assistance in obtaining Keio and ASKA collection strains as well as W. Navarre for helpful discussions.
Author information
Authors and Affiliations
Contributions
S.J.H. designed, performed and analysed most of the biological experiments and prepared the manuscript and figures. N.W. and A.S. performed and analysed the crystallography. M.S.S. performed the microscopy. K.M. performed and analysed the RT–qPCR. F.K. performed the permeability analysis, helped construct plasmids and identified reduced-toxicity TseH mutants. B.B. performed and analysed the pull-down assays. L.L. analysed the genome-sequencing data. A.P. and M.L. helped construct the strains and plasmids. N.W., A.S. and T.G.D. contributed to the manuscript revision. T.G.D. conceived the project and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
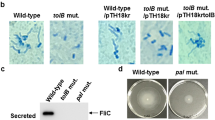
Extended Data Fig. 1 Further analyses of TseH killing, delivery, and structure.
a. Relative survival (killing by V. cholerae V52 TseHWT / TseHH64A) of E. coli, A. hydrophila, Edwardsiella sp., A. baylyi ADP1 (ΔT6SS), or V. parahaemolyticus RIMD2210633 strains. Welch’s one-way ANOVA with Dunnett’s multiple comparisons test comparing each sample to E. coli MG1655; *, p < 0.05; **, p < 0.01; ns, not significant. b. Relative survival (killing by TseHWT strain relative to killing by TseHH64A strain) of A. dhakensis expressing TsiH or vector only control. Unpaired 2-tailed t-test; ***, p < 0.001. c. Survival of A. dhakensis after killing by TseHH64A strain expressing TseH or vector only. Vector control data resembles no-plasmid data shown in Fig. 1a and was only repeated in duplicate. Unpaired 2-tailed t-test with Welch’s correction; **, p < 0.01. d. Extension of Fig. 1b in the main text. Survival of A. dhakensis prey after killing by ΔPAAR2 strain expressing plasmid-borne TseH and full-length or C-terminal truncations of PAAR2. For PAAR2: V, vector; numbers, PAAR2 truncated to indicated amino acid length; Δin, codons 146–156 removed leaving downstream residues intact. One-way ANOVA with Dunnett’s multiple comparisons test comparing to the sample expressing TseH and Vector control of PAAR2; ***, p < 0.001; ns, not significant. For all graphs, the mean and standard deviation are shown. Dots show individual replicates. e. Western blot showing whole cell lysate or eluted fraction after His-tag pull-down. BL21 DE3 E. coli expressed Flag-tagged TseH in combination with either His-tagged PAAR2 or Spy as a non-binding control. Number at left indicated ladder band size in kDa. Data is representative of two independent replicates. f. Circular permutation of catalytic residues causes swapping of N- and C-terminal lobes in the structure of TseH compared to Tse1 (PDB: 4EOB18), the other T6SS effector that belongs to NlpC/P60 family has reversed N- and C-lobe structure in comparison to TseH. g. Restricted access to the catalytic cysteine in certain peptidoglycan endopeptidases. The surface representations of SaCwlT and TseH show that the conformational changes in residues surrounding the active site are necessary for the substrate binding21,22. For all graphs, the mean and standard deviation are shown. Dots show individual replicates. TseHWT, V. cholerae V52 with all anti-bacterial effectors inactivated except TseH; TseHH64A, V52 with all anti-bacterial effectors inactivated including TseH.
Extended Data Fig. 2
Identified mutations that reduce TseH toxicity.
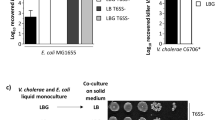
Extended Data Fig. 3 Additional E. coli mutants susceptible to T6SS-delivered TseH.
a. Disk diffusion induction of plasmid-borne TAT-tagged TseH expression in bacterial lawns of E. coli MG1655. WT (top) or H64A mutant (bottom) TseH are compared. Right discs contain the inducer of expression, arabinose (Ara), and left discs contain the repressor, glucose (Glu). Representative of three independent replicates. b. As in a, comparing induction of plasmid-borne TAT-tagged TseHWT expression in E. coli MG1655 or A. dhakensis (ΔT6SS). Similar data for TseHWT in E. coli has been shown previously and is shown again here for comparison4. c. Relative survival (killing by TseHWT / TseHH64A) of E. coli WT or knockout mutants from indicated categories. One-way ANOVA with Dunnett’s multiple comparisons test comparing each sample to WT; **, p < 0.01; ***, p < 0.001; ns, not significant. Data for WT (E. coli) is shown in Fig. 2 and is shown again here for comparison. Aux., auxotrophs. d. RT-qPCR comparing expression of genes in WT E. coli prey after T6SS competition assay with TseHWT or TseHH64A killer strains. Value with TseHH64A killer was set to 1 to show fold induction in response to TseH activity upon delivery by the T6SS. One-way ANOVA (with samples shown in Fig. 2d) with Sidak’s multiple comparisons test; ns, not significant. For both graphs, the mean and standard deviation are shown. Dots show individual replicates. TseHWT, V. cholerae V52 with all anti-bacterial effectors inactivated except TseH; TseHH64A, V52 with all anti-bacterial effectors inactivated including TseH.
Extended Data Fig. 4 Additional data for RcsA and BaeR regulon overexpression, and effect of Spy overexpression on permeability.
a. Disk diffusion induction of plasmid-borne TAT-tagged TseH in bacterial lawns of E. coli BW25113 containing RcsA or vector only plasmids. IPTG was added throughout the lawn to induce RcsA. Discs contain arabinose to induce TAT-TseH expression. Representative of three independent replicates. b. Relative survival (killing by TseHWT / TseHH64A) of E. coli ΔdegP mutant or A. dhakensis with vector (Vec) or overexpressing genes from the BaeR regulon. One-way ANOVA with Sidak’s multiple comparisons test comparing samples to their respective empty vector controls; *, p < 0.05; **, p < 0.01; ns, not significant. Vector controls are shown in Fig. 2 and are shown again here for comparison. Mean and standard deviations are shown. Dots show individual replicates. pSpyΔSec, plasmid expressing Spy with its Sec secretion tag deleted. c. Images of ΔbaeR E. coli overexpressing plasmid-borne TAT-TseH and either Spy or vector-only controls. The phase-contrast and propidium iodide(PI) channels are merged. Scale bar shows 5 μm. Representative of three independent replicates. d. Quantification showing the percent of total cells showing PI + fluorescence. N number at top indicates total cells counted. Dots show mean results from three independent experiments (used to calculate statistics) and error bars show one standard deviation. Unpaired 2-tailed t-test, p = 0.0513.
Extended Data Fig. 5 Additional data for ΔwigR strain T6SS susceptibility, immunity gene expression, VPS-mediated aggregation, and Aeromonas species do not encode RcsCDB, BaeSR, or WigKR.
Relative survival (WT V52 / TseHH64A killer strain) of V. cholerae V52 (T6SS+ background) prey (a.) or V. cholerae C6706 prey (b.) with intact (+), deleted (Δ), or complemented (Δ,p) wigR. Stationary (Stat.) and log phase prey are shown. One-way ANOVA with Sidak’s multiple comparisons test; ***, p < 0.001; **, p < 0.01; *, p < 0.05; ns, not significant. c. RT-qPCR measuring expression of T6SS immunity genes in ΔwigR V. cholerae V52 relative to in the wigR + strain (both in the ΔT6SS background). For all graphs, the mean and standard deviation are shown. Dots show individual replicates. d. Image showing settling of V. cholerae V52 grown at 37 °C or at 30 °C to induce VPS synthesis. Image after vortexing to disrupt aggregates is also shown for comparison (lower panel). Representative of three independent replicates. e. KEGG ortholog analysis across Gammaproteobacteria. KEGG Modules were compared for RcsCDB and BaeSR. WigK and WigR orthologs were assessed by sequence homology to VCA0565 and VCA0566 using an SW-score cutoff of 50% of maximum. Data is shown as the percentage of strains within each taxonomic group that contain an ortholog of all genes in the loci. Number in brackets indicates the number of strains in the taxonomic group in the KEGG database.
Extended Data Fig. 6
Table of data collection, phasing and refinement statistics for MAD (SeMet) structures.
Supplementary information
Supplementary Information
Supplementary Table 1: Strains and plasmids.
Supplementary Video 1
To visualize the effect of TseH on the cell wall, this video shows time-lapse fluorescence microscopy of E. coli cells expressing TseH (left), VgrG3 (middle) or TseL (right) effectors tagged to the periplasmic signal TAT. Cells were stained with 100 µg mL−1 of the PG-specific dye wheat germ agglutinin and spotted onto an agarose pad containing either 0.2% L-arabinose (TseH) or 1 mM IPTG (VgrG3 and TseL). Images were acquired every 20 sec during 25 min. Movie plays at a rate of 10 frames per second. Top row is a merge of phase and green (wheat germ agglutinin) channels and the bottom row shows green channel only. Scale bars, 5 µm. When expressing either TseH or the known cell-wall-targeting effector VgrG3, cells showed typical blebbing followed by PG sacculus deformation and cell lysis, whereas the effector TseL caused retraction and leaking of cytoplasmic content without obvious effect on PG. Video is representative of three independent replicates.
Source data
Source Data Fig. 1
Data and statistical source data used for Fig. 1.
Source Data Fig. 2
Data and statistical source data used for Fig. 2.
Source Data Fig. 3
Data and statistical source data used for Fig. 3.
Source Data Fig. 4
Data and statistical source data used for Fig. 4.
Source Data Fig. 5
Data and statistical source data used for Fig. 5.
Source Data Extended Data Fig. 1
Data and statistical source data used for Extended Data Fig. 1.
Source Data Extended Data Fig. 1
Uncropped western blots shown in Extended Data Fig. 1e.
Source Data Extended Data Fig. 3
Data and statistical source data used for Extended Data Fig. 3c,d. Please note that this is the same data as in Source Data for Fig. 2.
Source Data Extended Data Fig. 4
Data and statistical source data used for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Data and statistical source data used for Extended Data Fig. 5.
Rights and permissions
About this article
Cite this article
Hersch, S.J., Watanabe, N., Stietz, M.S. et al. Envelope stress responses defend against type six secretion system attacks independently of immunity proteins. Nat Microbiol 5, 706–714 (2020). https://doi.org/10.1038/s41564-020-0672-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-0672-6
This article is cited by
-
Capsules and their traits shape phage susceptibility and plasmid conjugation efficiency
Nature Communications (2024)
-
Competition quenching strategies reduce antibiotic tolerance in polymicrobial biofilms
npj Biofilms and Microbiomes (2024)
-
Collective protection against the type VI secretion system in bacteria
The ISME Journal (2023)
-
The RIX domain defines a class of polymorphic T6SS effectors and secreted adaptors
Nature Communications (2023)
-
Bacterial defences: mechanisms, evolution and antimicrobial resistance
Nature Reviews Microbiology (2023)