Abstract
Inflammasomes are signalling platforms that are assembled in response to infection or sterile inflammation by cytosolic pattern recognition receptors. The consequent inflammasome-triggered caspase-1 activation is critical for the host defence against pathogens. During infection, NLRP3, which is a pattern recognition receptor that is also known as cryopyrin, triggers the assembly of the inflammasome-activating caspase-1 through the recruitment of ASC and Nek7. The activation of the NLRP3 inflammasome is tightly controlled both transcriptionally and post-translationally. Despite the importance of the NLRP3 inflammasome regulation in autoinflammatory and infectious diseases, little is known about the mechanism controlling the activation of NLRP3 and the upstream signalling that regulates the NLRP3 inflammasome assembly. We have previously shown that the Rho-GTPase-activating toxin from Escherichia coli cytotoxic necrotizing factor-1 (CNF1) activates caspase-1, but the upstream mechanism is unclear. Here, we provide evidence of the role of the NLRP3 inflammasome in sensing the activity of bacterial toxins and virulence factors that activate host Rho GTPases. We demonstrate that this activation relies on the monitoring of the toxin’s activity on the Rho GTPase Rac2. We also show that the NLRP3 inflammasome is activated by a signalling cascade that involves the p21-activated kinases 1 and 2 (Pak1/2) and the Pak1-mediated phosphorylation of Thr 659 of NLRP3, which is necessary for the NLRP3–Nek7 interaction, inflammasome activation and IL-1β cytokine maturation. Furthermore, inhibition of the Pak–NLRP3 axis decreases the bacterial clearance of CNF1-expressing UTI89 E. coli during bacteraemia in mice. Taken together, our results establish that Pak1 and Pak2 are critical regulators of the NLRP3 inflammasome and reveal the role of the Pak–NLRP3 signalling axis in vivo during bacteraemia in mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information or from the corresponding author on reasonable request. Source data are provided with this paper.
References
Martin, G. S., Mannino, D. M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Vance, R. E., Isberg, R. R. & Portnoy, D. A. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe 6, 10–21 (2009).
Stuart, L. M., Paquette, N. & Boyer, L. Effector-triggered versus pattern-triggered immunity: how animals sense pathogens. Nat. Rev. Immunol. 13, 199–206 (2013).
Flatau, G. et al. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387, 729–733 (1997).
Schmidt, G. et al. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-1. Nature 387, 725–729 (1997).
Aktories, K. & Barbieri, J. Bacterial cytotoxins: targeting eukaryotic switches. Nat. Rev. Microbiol. 3, 397–410 (2005).
Galán, J. E. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5, 571–579 (2009).
Bruno, V. M. et al. Salmonella Typhimurium type III secretion effectors stimulate innate immune responses in cultured epithelial cells. PLoS Pathog. 5, e1000538 (2009).
Munro, P. et al. Activation and proteasomal degradation of Rho GTPases by cytotoxic necrotizing factor-1 elicit a controlled inflammatory response. J. Biol. Chem. 279, 35849–35857 (2004).
Boquet, P. & Lemichez, E. Bacterial virulence factors targeting Rho GTPases: parasitism or symbiosis? Trends Cell Biol. 13, 238–246 (2003).
Diabate, M. et al. Escherichia coli α-hemolysin counteracts the anti-virulence innate immune response triggered by the Rho GTPase activating toxin CNF1 during bacteremia. PLoS Pathog. 11, e1004732 (2015).
Xu, H. et al. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 513, 237–241 (2014).
Groslambert, M. & Py, B. F. Spotlight on the NLRP3 inflammasome pathway. J. Inflamm. Res. 11, 359–374 (2018).
Yang, Y., Wang, H., Kouadir, M., Song, H. & Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10, 128 (2019).
Gao, W., Yang, J., Liu, W., Wang, Y. & Shao, F. Site-specific phosphorylation and microtubule dynamics control pyrin inflammasome activation. Proc. Natl Acad. Sci. USA 113, E4857–E4866 (2016).
Park, Y. H., Wood, G., Kastner, D. L. & Chae, J. J. Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat. Immunol. 17, 914–921 (2016).
Tzeng, T. C. et al. A fluorescent reporter mouse for inflammasome assembly demonstrates an important role for cell-bound and free ASC specks during in vivo Infection. Cell Rep. 16, 571–582 (2016).
Sester, D. P. et al. Assessment of inflammasome formation by flow cytometry. Curr. Protoc. Immunol. 114, 14.40.1–14.40.29 (2016).
Lamkanfi, M. & Dixit, V. M. In retrospect: the inflammasome turns 15. Nature 548, 534–535 (2017).
He, Y., Hara, H. & Núñez, G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem. Sci. 41, 1012–1021 (2016).
Shi, H., Murray, A. & Beutler, B. Reconstruction of the mouse inflammasome system in HEK293T cells. Bio. Protoc. 6, e1986 (2016).
Keestra, A. M. et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature 496, 233–237 (2013).
Doye, A. et al. CNF1 exploits the ubiquitin-proteasome machinery to restrict Rho GTPase activation for bacterial host cell invasion. Cell 111, 553–564 (2002).
Boyer, L. et al. Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway. Immunity 35, 536–549 (2011).
Manser, E., Leung, T., Salihuddin, H., Zhao, Z. S. & Lim, L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 (1994).
Wells, C. M. & Jones, G. E. The emerging importance of group II PAKs. Biochem. J. 425, 465–473 (2010).
Semenova, G. & Chernoff, J. Targeting PAK1. Biochem. Soc. Trans. 45, 79–88 (2017).
Song, N. et al. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol. Cell 68, 185–197 (2017).
Sharif, H. et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature 570, 338–343 (2019).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Kelly, M. L. & Chernoff, J. Mouse models of PAK function. Cell Logist. 2, 84–88 (2012).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
He, W. T. et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015).
Broz, P., Pelegrín, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Rühl, S. et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018).
Evavold, C. L. et al. The pore-forming protein gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44 (2018).
Monteleone, M. et al. Interleukin-1β maturation triggers its relocation to the plasma membrane for gasdermin-D-dependent and -independent secretion. Cell Rep. 24, 1425–1433 (2018).
Pandori, W. J. et al. Toxoplasma gondii activates a Syk-CARD9-NF-κB signaling axis and gasdermin D-independent release of IL-1β during infection of primary human monocytes. PLoS Pathog. 15, e1007923 (2019).
Muessel, M. J., Harry, G. J., Armstrong, D. L. & Storey, N. M. SDF-1α and LPA modulate microglia potassium channels through rho GTPases to regulate cell morphology. Glia 61, 1620–1628 (2013).
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Lopes Fischer, N., Naseer, N., Shin, S. & Brodsky, I. E. Effector-triggered immunity and pathogen sensing in metazoans. Nat. Microbiol. 5, 14–26 (2020).
Aubert, D. F. et al. A Burkholderia type VI effector deamidates Rho GTPases to activate the pyrin inflammasome and trigger inflammation. Cell Host Microbe 19, 664–674 (2016).
Medici, N. P., Rashid, M. & Bliska, J. B. Characterization of pyrin dephosphorylation and inflammasome activation in macrophages as triggered by the yersinia effectors YopE and YopT. Infect. Immun. 87, e00822-18 (2019).
Cabral, V. P., Andrade, C. A., Passos, S. R., Martins, M. F. & Hökerberg, Y. H. Severe infection in patients with rheumatoid arthritis taking anakinra, rituximab, or abatacept: a systematic review of observational studies. Rev. Bras. Reumatol. Engl. Ed. 56, 543–550 (2016).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Mulvey, M. A., Schilling, J. D. & Hultgren, S. J. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69, 4572–4579 (2001).
Buetow, L., Flatau, G., Chiu, K., Boquet, P. & Ghosh, P. Structure of the Rho-activating domain of Escherichia coli cytotoxic necrotizing factor 1. Nat. Struct. Biol. 8, 584–588 (2001).
Doye, A., Boyer, L., Mettouchi, A. & Lemichez, E. Ubiquitin-mediated proteasomal degradation of Rho proteins by the CNF1 toxin. Methods Enzymol. 406, 447–456 (2006).
Matsuzawa, T., Kashimoto, T., Katahira, J. & Horiguchi, Y. Identification of a receptor-binding domain of Bordetella dermonecrotic toxin. Infect. Immun. 70, 3427–3432 (2002).
Kubori, T. & Galán, J. E. Temporal regulation of salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115, 333–342 (2003).
Lagrange, B. et al. Human caspase-4 detects tetra-acylated LPS and cytosolic Francisella and functions differently from murine caspase-11. Nat. Commun. 9, 242 (2018).
Martinon, F., Pétrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
McDaniel, A. S. et al. Pak1 regulates multiple c-Kit mediated Ras-MAPK gain-in-function phenotypes in Nf1+/− mast cells. Blood 112, 4646–4654 (2008).
Stutz, A. et al. NLRP3 inflammasome assembly is regulated by phosphorylation of the pyrin domain. J. Exp. Med. 214, 1725–1736 (2017).
Acknowledgements
We thank P. Auberger, A. Baumler, I. Brodsky, J. Chernoff, D. Golenbock, T. Henry, M. Keestra-Gounder, E. Lemichez, E. Manser, E. Meunier, V. Petrilli, D. Pisani, J.-E. Ricci, G. Robert, P.-M. Roger, L. Stuart, P. Vandenabeele, S. Ivanov and L. Yvan-Charvet for sharing materials or discussions; A.-S. Dufour, E. Garcia, M. Irondelle and J. Murdaca for technical assistance; members of the Innate Sensors Community (InnaSCo) for sharing tools; A. Cuttriss and staff at the Office of International Scientific Visibility of Université Côte d’Azur for professional language editing; staff at the Etablissement Français du Sang of Marseille for providing human blood from human healthy donors; and staff at the C3M facilities (animal, genomic, cytometry and imaging) and the Harvard Taplin mass spectrometry core. The mouse strain used for this research project, B6.129S2-Pak1tm1Cher/Mmnc (RRID, MMRRC_031838-UNC) was obtained from the Mutant Mouse Resource and Research Center (MMRRC) at University of North Carolina at Chapel Hill, an NIH-funded strain repository, and was donated to the MMRRC by J. Chernoff, Fox Chase Cancer Center. This work was supported by grants from the ANR (ANR-17-CE15-0001), Investments for the Future programs LABEX SIGNALIFE ANR-11-LABX-0028-01, IDEX UCAJEDI ANR-15-IDEX-01, ARC (RAC15014AAA), Université Côte d’Azur, Infectiopole sud and REDPIT. B.F.P. is supported by ERC (ERC-2013-CoG_616986). A.M. is supported by a fellowship from FRM; C.T. by a fellowship from Ville de Nice; and O.D. by a fellowship from INSERM and Université Côte d’Azur.
Author information
Authors and Affiliations
Contributions
O.D. and A.D. designed, performed and analysed most of the experiments with input from C.T., C.L., A.J., A.G., A.M., P.C., A.R. and S.M.; J.C. and R.R. provided advice on the mice infection model. E.V., R.R.G., D.C., B.F.P., A.R., P.H.V.S. and M.L. provided tools and advice on NLRP3 inflammasome regulation. P.M. generated NLRP3 mutants and, with G.M. and O.D., performed and analysed most of the in vivo experiments. O.V. performed virulence factor and toxin subcloning, protein purifications, the in vitro kinase assay and analysed the mass spectrometry results. L.B. conceived the project, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Microbiology thanks Igor Brodsky, Gad Frankel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 CNF1 triggers Caspase-1 activation and ASC specks formation.
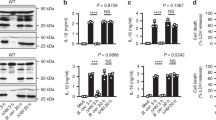
a, BMDMs isolated from BALB/c mice were either treated with vehicle (control) or CNF1 (500 ng ml–1), inactive catalytic mutant CNF1 C866S (500 ng/mL) for 6 h, or Nigericin (5 μM) for 30 min. Active Caspase-1 was revealed with FAM-FLICA (green), ASC was stained using an anti-ASC antibody (Texas Red), nuclei and actin filament were stained with Hoechst and phalloidin-Alexa 647 respectively. Cells were analyzed by confocal microscopy. Arrows indicates FAM-FLICA dots that colocalize with the ASC staining. Scale bar: 10μm. b, BMDM isolated from C57BL/6 J mice constitutively expressing ASC-citrine fusion protein (R26-CAG-ASC-citrine) were transfected with the indicated siRNA for 72 h prior to 6 h of CNF1 treatment (500 ng/mL) or treated with vehicle (control). Percent of cells with ASC specks. Data are expressed as the mean ± SEM. Each dot represents 105 cells (n = 2 biologically independent samples). c, BMDM isolated from C57BL/6 J mice constitutively expressing ASC-citrine fusion protein (R26-CAG-ASC-citrine) were treated 6 h with CNF1 (500 ng/mL) or Nigericin (5 μM) for 30 min or vehicle (control). Cells were analyzed for ASC speck formation by flow cytometry as indicated, doublets were excluded using SSC-A and SSC-H plot, cells with a high expression of ASC-citrine were gated and then analyzed for ASC-citrine area (ASC-citrine-A) and ASC-citrine height (ASC-citrine-H). Cells with ASC specks are defined with a higher ASC-H:ASC-A ratio. Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 2 NLRP3 inflammasome activation by CNF1 does not induce pyroptosis.
a, BMDMs isolated from C57BL/6 J mice were treated with vehicle (control), Nigericin (5 μM) or CNF1 (500 ng/mL or 5 μg/mL) with or without LPS (100 ng/mL). Propidium iodide (PI) uptake was monitored over time (red object count) by real time imaging. Data are expressed as mean ± SD. 104 cells were analyzed for each replicate (n = 4 independent wells). b, BMDMs isolated from C57BL/6 J mice were treated with vehicle (control, n = 6 independent experiments), LPS (100 ng/mL, n = 4 independent experiments), LPS and CNF1 (500 ng/mL, n = 6 independent experiments) or LPS and Nigericin (5 μM, n = 4 independent experiments), and LDH release was assessed. Data are expressed as the mean ± SEM. Statistical analyses were performed using a two-tailed nonparametric Mann Whitney test. c, BMDMs isolated from C57BL/6 J mice were treated either with Nigericin (5 μM) for 30 min or CNF1 (0.5, 1 or 5 μg/mL) for 8 h and GSDMD cleavage in cell lysates is shown. d, BMDMs isolated from C57BL/6 J wild-type or CASP1, CASP11, PYCARD (coding for ASC) or GSDMD knock-out mice were untreated or treated with CNF1 (500 ng/mL) for 6 h and were analyzed for Caspase-1 activation using the FAM-FLICA probe. Data are expressed as the mean ± SEM. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. Each dot represents 100 cells (n = 700 cells). e, BMDMs isolated from wild-type or GSDMD knock-out mice were treated with CNF1 (500 ng/mL) and LPS (100 ng/mL) for 8 h as indicated. Supernatants and cell lysates were analyzed by immunoblot. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 3 CNF1-triggered inflammasome activation depends on NLRP3, Nek7 and K+ efflux.
a,b, BMDMs isolated from C57BL/6 J mice were transfected with siRNA-targeting NLRP3 (a), siRNA-targeting Nek7 (b), or control non-targeting siRNA for 72 h before treatment with CNF1 (500 ng/mL) and/or LPS (100 ng/mL) for 8 h. Supernatants and cell lysates were analyzed by immunoblot. c,d, BMDMs isolated from C57BL/6 J mice (c) or iBMDMs (d) were treated with the indicated KCl concentration and CNF1 (500 ng/mL) for 8 h. c, Supernatants and cell lysates were analyzed by immunoblot, or (d) cell lysates were analyzed using a GST–Pak-RBD pull-down assay. The Rac associated with the GST-Pak-RBD beads is indicated as Rac-GTP. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 4 Toxins mediated Rho GTPases activation but not inhibition trigger the NLRP3 inflammasome.
a, BMDMs isolated from wild-type or NLRP3 knock out C57BL/6 J mice were treated with DNT (1 µg/mL) for 8 h. Supernatants and cell lysates were analyzed by immunoblot. b-c, HEK293T cells were transfected as indicated with plasmids encoding NLRP3 inflammasome components (myc-NLRP3, ASC-GFP, mCaspase-1) and pro-IL-1β-Flag together with (b) myc-DNT, HA-YopE or myc-Dbl495–826 or (c) transfected with SopE-HA and treated with MG132 to block SopE degradation (10 μM). Supernatants and cell lysates were analyzed by immunoblot. d, BMDMs isolated from C57BL/6 J mice were treated with IPA-3 (5 μM) or MCC950 (1 μM) for 45 min prior to 8 h of DNT treatment (1 μg/mL). Supernatants and cell lysates were analyzed by immunoblot. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 5 Inhibition of Pak1 diminishes NLRP3 activation by Nigericin.
a,b, BMDMs isolated from C57BL/6 J mice were treated with MCC950 (1 μM) or IPA-3 (5 μM) for 45 min prior to Nigericin (5 μM) treatment for 30 min. Supernatants and cell lysates were analyzed by (a) immunoblot and (b) supernatants were analyzed for LDH release (n = 3 biologically independent experiments). Statistical analyses were performed using a two-tailed nonparametric Mann Whitney test. n = 3 biologically independent samples were analyzed. c, BMDMs isolated from C57BL/6 J mice were treated for 72 h with non-targeting (CT) or Pak1-targeting siRNA before treatment with Nigericin (5 μM) for 30 min. Supernatants and cell lysates were analyzed by immunoblot. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown. Data are expressed as the mean ± SEM.
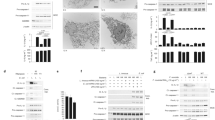
Extended Data Fig. 6 Mass spectrometry analysis of Pak1 triggered NLRP3 phosphorylation.
a-c, Fragmentation spectra of human NLRP3 peptides showing phosphorylation of Ser-163, Ser-198 and Thr-659. d, Representation of NLRP3 domain structure and sequence alignment of NLRP3 ortholog peptides surrounding phosphorylated residues identified by mass spectrometry. The phosphorylated residues are in bold red.
Extended Data Fig. 7 Conservation of the Pak-NLRP3 axis in Human monocyte-derived macrophages.
a-b, Human monocyte-derived macrophages (hMDMs) were pretreated with vehicle, MCC950 (1 μM) or IPA-3 (5 μM) for 45 min before CNF1 (500 ng/mL) treatment for 6 h. Active Caspase-1 was stained with FAM-FLICA (green), NLRP3 (red) and nuclei (blue) were stained for immunofluorescence and confocal microscopy imaging. Arrows indicates FAM-FLICA dots that colocalize with NLRP3. Scale bar: 20 μm. b, quantification of FAM-FLICA positive cells. Data are expressed as the mean ± SEM. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. Each dot represents 100 cells (n = 1800 cells). Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 8 The NLRP3 T659A mutant inhibit the IL-1β maturation triggered by SopE and DNT.
a, HEK293T cells were transfected with plasmids encoding NLRP3 inflammasome components (ASC-GFP, mCaspase-1) and pro-IL-1β-Flag and either myc-NLRP3 (WT) or myc-NLRP3 T659A together with SopE-HA and treated with MG132 (10 μM) to block SopE degradation. Supernatants and cell lysates were analyzed by immunoblot. b, NLRP3 knock-out iBMDMs reconstituted either with NLRP3 or NLRP3 T659A were treated with vehicle or LPS (100 ng/mL) and DNT (1 μg/mL) for 8 h. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown.
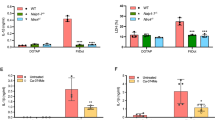
Extended Data Fig. 9 Macrophages expressing the NLRP3 T659A mutant have an impaired Nigericin- triggered IL-1β maturation.
a-b, iBMDMs stably expressing either NLRP3 or NLRP3 T659A were treated with Nigericin (5 μM) for 30 min. Supernatants and cell lysates were analyzed by immunoblot and by ELISA for IL-1β (n = 4 biologically independent samples) and TNF-α (n = 3 biologically independent samples). Data are expressed as the mean ± SEM. Statistical analyses were performed using a two-tailed unpaired Student’s t-test. The numbers on the side of the immunoblots indicate molecular weight (kDa). Experiments were repeated at least three times, and representative data are shown.
Extended Data Fig. 10 E. coli CNF1− clearing is not affected by Pak1 or NLRP3 inhibition and E. coli CNF1+ clearing does not rely on GSDMD.
a-d, Wild-type or knock-out mice were infected intravenously with isogenic CNF1-deleted E. coli (E. coli CNF1−) or CNF1 expressing E. coli (E. coli CNF1+). a, Wild-type mice were injected intraperitoneally with 10 mg/kg AZ13711265 or 50 mg/kg MCC950 or vehicle once a day and were infected intravenously with isogenic CNF1-deleted E. coli (E. coli CNF1−) prior to the collection of peripheral blood at 4 h, 24 h and 48 h for measurement of bacteraemia (n = 5 mice per group). b, Wild-type or NLRP3 knock-out C57BL/6 J mice were infected intravenously with CNF1 expressing E. coli (E. coli CNF1+) prior to the collection of peripheral blood at 4 h, 24 h, 48 h, 72 h and 96 h for measurement of bacteraemia (n = 6 per group). c, Wild-type (n = 6 mice) or NLRP3 knock-out C57BL/6 J mice (n = 4 mice) were infected intravenously with isogenic CNF1-deleted E. coli (E. coli CNF1−) prior to the collection of peripheral blood at 4 h, 24 h and 48 h for measurement of bacteraemia (n = 6 per group). d, Wild-type (n = 6 mice) or GSDMD knock-out C57BL/6 J mice (n = 6 mice) were infected intravenously with E. coli CNF1+ prior to the collection of peripheral blood at 4 h, 24 h and 48 h for measurement of bacteraemia. Experiments were repeated two times and representative data are shown. Data are expressed as the geometric mean ± 95 CI.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3 and Table 1.
Source data
Source Data Fig. 1
Unprocessed blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed blots.
Source Data Fig. 3
Unprocessed blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed blots.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed blots.
Source Data Extended Data Fig. 4
Unprocessed blots.
Source Data Extended Data Fig. 5
Unprocessed blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed blots.
Source Data Extended Data Fig. 9
Unprocessed blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Rights and permissions
About this article
Cite this article
Dufies, O., Doye, A., Courjon, J. et al. Escherichia coli Rho GTPase-activating toxin CNF1 mediates NLRP3 inflammasome activation via p21-activated kinases-1/2 during bacteraemia in mice. Nat Microbiol 6, 401–412 (2021). https://doi.org/10.1038/s41564-020-00832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-00832-5
This article is cited by
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
Bergapten inhibits NLRP3 inflammasome activation and pyroptosis via promoting mitophagy
Acta Pharmacologica Sinica (2023)
-
Optineurin links Hace1-dependent Rac ubiquitylation to integrin-mediated mechanotransduction to control bacterial invasion and cell division
Nature Communications (2022)
-
TREM2/β-catenin attenuates NLRP3 inflammasome-mediated macrophage pyroptosis to promote bacterial clearance of pyogenic bacteria
Cell Death & Disease (2022)
-
p21-activated kinase 1 (PAK1) as a therapeutic target for cardiotoxicity
Archives of Toxicology (2022)