Abstract
Bacteria are encapsulated by a peptidoglycan cell wall that is essential for their survival1. During cell wall assembly, a lipid-linked disaccharide–peptide precursor called lipid II is polymerized and cross-linked to produce mature peptidoglycan. As lipid II is polymerized, nascent polymers remain membrane-anchored at one end, and the other end becomes cross-linked to the matrix2,3,4. How bacteria release newly synthesized peptidoglycan strands from the membrane to complete the synthesis of mature peptidoglycan is a long-standing question. Here, we show that a Staphylococcus aureus cell wall hydrolase and a membrane protein that contains eight transmembrane helices form a complex that may function as a peptidoglycan release factor. The complex cleaves nascent peptidoglycan internally to produce free oligomers as well as lipid-linked oligomers that can undergo further elongation. The polytopic membrane protein, which is similar to a eukaryotic CAAX protease, controls the length of these products. A structure of the complex at a resolution of 2.6 Å shows that the membrane protein scaffolds the hydrolase to orient its active site for cleaving the glycan strand. We propose that this complex functions to detach newly synthesized peptidoglycan polymer from the cell membrane to complete integration into the cell wall matrix.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Transposon sequencing data (BioProject accession number PRJNA573479) are available in the NCBI BioProject database. The coordinates and structure factors for the SagB–SpdC structure have been deposited with the PDB under the accession number 6U0O. Source data are provided with this paper.
Code availability
Scripts for this analysis are available at https://github.com/SuzanneWalkerLab/TnSeqMOAPrediction.
References
Silhavy, T. J., Kahne, D. & Walker, S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2, a000414 (2010).
Vollmer, W., Blanot, D. & de Pedro, M. A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 32, 149–167 (2008).
Meeske, A. J. et al. SEDS proteins are a widespread family of bacterial cell wall polymerases. Nature 537, 634–638 (2016).
Taguchi, A. et al. FtsW is a peptidoglycan polymerase that is functional only in complex with its cognate penicillin-binding protein. Nat. Microbiol. 4, 587–594 (2019).
Sham, L. T. et al. MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis. Science 345, 220–222 (2014).
Ruiz, N. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc. Natl Acad. Sci. USA 105, 15553–15557 (2008).
Sauvage, E., Kerff, F., Terrak, M., Ayala, J. A. & Charlier, P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32, 234–258 (2008).
Yunck, R., Cho, H. & Bernhardt, T. G. Identification of MltG as a potential terminase for peptidoglycan polymerization in bacteria. Mol. Microbiol. 99, 700–718 (2016).
Santiago, M. et al. Genome-wide mutant profiling predicts the mechanism of a lipid II binding antibiotic. Nat. Chem. Biol. 14, 601–608 (2018).
Santiago, M. et al. A new platform for ultra-high density Staphylococcus aureus transposon libraries. BMC Genom. 16, 252 (2015).
Georgopapadakou, N. H., Smith, S. A. & Bonner, D. P. Penicillin-binding proteins in a Staphylococcus aureus strain resistant to specific beta-lactam antibiotics. Antimicrob. Agents Chemother. 22, 172–175 (1982).
Wheeler, R. et al. Bacterial cell enlargement requires control of cell wall stiffness mediated by peptidoglycan hydrolases. mBio 6, e00660 (2015).
Chan, Y. G., Frankel, M. B., Missiakas, D. & Schneewind, O. SagB glucosaminidase is a determinant of Staphylococcus aureus glycan chain length, antibiotic susceptibility, and protein secretion. J. Bacteriol. 198, 1123–1136 (2016).
Pasquina-Lemonche, L. et al. The architecture of the Gram-positive bacterial cell wall. Nature 582, 294–297 (2020).
Grundling, A., Missiakas, D. M. & Schneewind, O. Staphylococcus aureus mutants with increased lysostaphin resistance. J. Bacteriol. 188, 6286–6297 (2006).
Poupel, O., Proux, C., Jagla, B., Msadek, T. & Dubrac, S. SpdC, a novel virulence factor, controls histidine kinase activity in Staphylococcus aureus. PLoS Pathog. 14, e1006917 (2018).
Schaefer, K., Matano, L. M., Qiao, Y., Kahne, D. & Walker, S. In vitro reconstitution demonstrates the cell wall ligase activity of LCP proteins. Nat. Chem. Biol. 13, 396–401 (2017).
Ye, X. Y. et al. Better substrates for bacterial transglycosylases. J. Am. Chem. Soc. 123, 3155–3156 (2001).
Qiao, Y. et al. Lipid II overproduction allows direct assay of transpeptidase inhibition by beta-lactams. Nat. Chem. Biol. 13, 793–798 (2017).
Pintar, S., Borisek, J., Usenik, A., Perdih, A. & Turk, D. Domain sliding of two Staphylococcus aureus N-acetylglucosaminidases enables their substrate-binding prior to its catalysis. Commun. Biol. 3, 178 (2020).
Perlstein, D. L., Zhang, Y., Wang, T. S., Kahne, D. E. & Walker, S. The direction of glycan chain elongation by peptidoglycan glycosyltransferases. J. Am. Chem. Soc. 129, 12674–12675 (2007).
Wang, T. S. et al. Primer preactivation of peptidoglycan polymerases. J. Am. Chem. Soc. 133, 8528–8530 (2011).
Welsh, M. A., Schaefer, K., Taguchi, A., Kahne, D. & Walker, S. The direction of chain growth and substrate preferences of SEDS-family peptidoglycan glycosyltransferases. J. Am. Chem. Soc. 141, 12994–12997 (2019).
El Ghachi, M. et al. Colicin M exerts its bacteriolytic effect via enzymatic degradation of undecaprenyl phosphate-linked peptidoglycan precursors. J. Biol. Chem. 281, 22761–22772 (2006).
Touze, T. et al. Colicin M, a peptidoglycan lipid-II-degrading enzyme: potential use for antibacterial means? Biochem. Soc. Trans. 40, 1522–1527 (2012).
Alcorlo, M., Martinez-Caballero, S., Molina, R. & Hermoso, J. A. Carbohydrate recognition and lysis by bacterial peptidoglycan hydrolases. Curr. Opin. Struct. Biol. 44, 87–100 (2017).
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4, 706–731 (2009).
Mihelic, M. et al. The mechanism behind the selection of two different cleavage sites in NAG-NAM polymers. IUCrJ 4, 185–198 (2017).
Qiao, Y. et al. Detection of lipid-linked peptidoglycan precursors by exploiting an unexpected transpeptidase reaction. J. Am. Chem. Soc. 136, 14678–14681 (2014).
Rebets, Y. et al. Moenomycin resistance mutations in Staphylococcus aureus reduce peptidoglycan chain length and cause aberrant cell division. ACS Chem. Biol. 9, 459–467 (2014).
Welsh, M. A. et al. Identification of a functionally unique family of penicillin-binding proteins. J. Am. Chem. Soc. 139, 17727–17730 (2017).
Schaefer, K., Owens, T. W., Kahne, D. & Walker, S. Substrate preferences establish the order of cell wall assembly in Staphylococcus aureus. J. Am. Chem. Soc. 140, 2442–2445 (2018).
Flores-Kim, J., Dobihal, G. S., Fenton, A., Rudner, D. Z. & Bernhardt, T. G. A switch in surface polymer biogenesis triggers growth-phase-dependent and antibiotic-induced bacteriolysis. eLife 8, e44912 (2019).
Coe, K. A. et al. Multi-strain Tn-Seq reveals common daptomycin resistance determinants in Staphylococcus aureus. PLoS Pathog. 15, e1007862 (2019).
Kato, F. & Sugai, M. A simple method of markerless gene deletion in Staphylococcus aureus. J. Microbiol. Methods 87, 76–81 (2011).
Do, T. et al. Staphylococcus aureus cell growth and division are regulated by an amidase that trims peptides from uncrosslinked peptidoglycan. Nat. Microbiol. 5, 291–303 (2020).
Lee, W. et al. Antibiotic combinations that enable one-step, targeted mutagenesis of chromosomal genes. ACS Infect. Dis. 4, 1007–1018 (2018).
Pang, T., Wang, X., Lim, H. C., Bernhardt, T. G. & Rudner, D. Z. The nucleoid occlusion factor Noc controls DNA replication initiation in Staphylococcus aureus. PLoS Genet. 13, e1006908 (2017).
Do, T. et al. Staphylococcus aureus cell growth and division are regulated by an amidase that trims peptides from uncrosslinked peptidoglycan. Nat. Microbiol. 5, 291–303 (2020).
Bertsche, U. et al. Interaction between two murein (peptidoglycan) synthases, PBP3 and PBP1B, in Escherichia coli. Mol. Microbiol. 61, 675–690 (2006).
Barrett, D. et al. Analysis of glycan polymers produced by peptidoglycan glycosyltransferases. J. Biol. Chem. 282, 31964–31971 (2007).
Lebar, M. D. et al. Forming cross-linked peptidoglycan from synthetic Gram-negative lipid II. J. Am. Chem. Soc. 135, 4632–4635 (2013).
Kuhner, D., Stahl, M., Demircioglu, D. D. & Bertsche, U. From cells to muropeptide structures in 24h: peptidoglycan mapping by UPLC-MS. Sci. Rep. 4, 7494 (2014).
Jackson, P. The use of polyacrylamide-gel electrophoresis for the high-resolution separation of reducing saccharides labelled with the fluorophore 8-aminonaphthalene-1,3,6-trisulphonic acid. Detection of picomolar quantities by an imaging system based on a cooled charge-coupled device. Biochem. J. 270, 705–713 (1990).
Kabsch, W. Xds. Acta Crystallogr. D 66, 125–132 (2010).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D 69, 1204–1214 (2013).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Manolaridis, I. et al. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature 504, 301–305 (2013).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Acknowledgements
We thank S. Moussa for his preliminary experiments investigating the roles of SpdC and SagB; and A. Kruse for discussions on crystallography. For this research, we used NE-CAT beamlines (GM103403), a Pilatus detector (RR029205) and an Eiger detector (OD021527) at the APS (DE-AC02-06CH11357). This research was supported by grant nos. GM076710 and U19 AI109764 to D.K. and S.W., and T32GM007753 to J.E.P.
Author information
Authors and Affiliations
Contributions
S.W., K.S., T.W.O. and J.E.P. designed experiments and analysed the data with input from D.K.; K.S. performed the biochemical experiments; K.S. and T.W.O. purified proteins and performed crystallographic experiments; J.E.P. analysed transposon sequencing data, constructed S. aureus mutant strains, performed the spot dilutions and performed the glycan strand experiment; S.W., K.S., T.W.O., J.E.P. and D.K. wrote the manuscript with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Hydrolase activities are unaffected by acidic conditions.
Peptidoglycan oligomers were prepared at pH 5.5 in sodium citrate buffer, and then incubated with the respective hydrolase. Cleavage patterns are similar to those observed at pH 6.5 (see main text Fig. 2).
Extended Data Fig. 2 The complex cleaves native PG oligomers to defined lengths similarly to synthetic PG oligomers.
To prepare linear glycan strands, extracted S. aureus Lipid II was incubated with transpeptidase-inactive PBP2S398G and then the SagB-SpdC complex or individual hydrolases (SagB or SagA). Reactions were quenched and products were then labeled with biotin-D-lysine (BDL) in the presence of the E. faecalis PBPX and visualized using a western blot method with HRP-streptavidin detection19,23,29.
Extended Data Fig. 3 The catalytic glutamate of SagB (E155), but not conserved CAAX protease residues in SpdC (E135, R139, H210)5, is necessary to complement shared cell wall phenotypes of sagB and spdC mutants.
Spot dilution series of S. aureus strains with mutations in sagB or spdC were plated on TSA or TSA containing tunicamycin or lysostaphin. Plates shown on the right are replicates of those on the left but the agar contains the inducer, anhydrotetracycline.
Extended Data Fig. 4 Glycan strand lengths are longer in the absence of spdC or sagB.
a, Sacculi were isolated from wild-type, ∆sagB, and ∆spdC S. aureus strains. The purified peptidoglycan was then treated with the endopeptidase lysostaphin, which cleaves the PG crosslinks, and the amidase LytA, which removes the remaining stem peptides. These denuded glycan strands were then labeled at the reducing end by reductive amination with the anionic fluorophore 8-aminonaphthalene-1,3,6-trisulfonic acid (ANTS), and separated by gel electrophoresis for in-gel imaging (UV excitation at 365 nm, visible emission). b, In lane 1, showing PG isolated from a wild-type strain, shorter glycan strands are visualized as a discrete ladder. In lanes 2 and 3, representing glycan strands of ∆sagB and ∆spdC respectively, this discrete ladder of short glycan strands is lost.
Extended Data Fig. 5 Nascent peptidoglycan is the preferred substrate of SagB-SpdC.
Cold glycan strands were prepared with radiolabeled wall teichoic acid (lanes 2–5) and incubated with a hydrolase. In the presence of either SagB (lane 3) or SagB-SpdC (lane 4), the ladder signal remained the same as a no treatment control (lane 2). There was reduced radiolabeled signal towards the top of the gel in both reactions; this signal represents a population of longer peptidoglycan oligomers and the fainter signal suggests less wall teichoic acid present. In contrast, mutanolysin was able to hydrolyze wall teichoic acid-modified glycan strands. To test whether SagB-SpdC cleavage interfered with wall teichoic acid ligation, cold oligomers were prepared and then incubated with radiolabeled wall teichoic precursor, LIIAWTA, and the wall teichoic acid ligase, TagT. The appearance of the radiolabeled ladder indicates that SagB-SpdC products were ligated with wall teichoic acid (lane 9).
Extended Data Fig. 6 Crosslinked PG is not a substrate for SagB-SpdC.
S. aureus Lipid II was co-incubated with wild-type PBP2, a hydrolase, and BDL for visualization. Reactions were quenched, halved, and then one portion was treated with lysostaphin. Crosslinked peptidoglycan oligomers do not readily enter the gel matrix (lane 1) and appear as a dark smear when crosslinks are cleaved by the endopeptidase lysostaphin (lane 4). In reactions containing SagB-SpdC, a low-molecular weight ladder of bands is present without lysostaphin (lane 2) or with lysostaphin (lane 5). This shows that these products, generated by SagB-SpdC, are not crosslinked as they were not changed with lysostaphin treatment. Notably, approximately 20% of peptidoglycan peptides are crosslinked by wild-type PBP2 in vitro36. The high molecular weight signals (smears) in lanes 5 and 6 are similar to the signal in lane 4, indicating that crosslinked PG is not cleaved by either SagB-SpdC or full-length SagB.
Extended Data Fig. 7 Representative electron density.
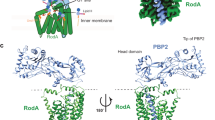
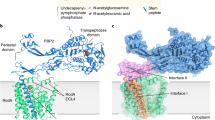
a–d, Simulated-annealing composite omit 2FO-FC electron density maps contoured at 1σ and carved 1.6 Å from the model, with SagB colored violet and SpdC colored green. a, Overall structure of SagB-SpdC viewed in the plane of the membrane. b, View of the transmembrane helices from the extracellular face. c, Active site groove of SagB, with sidechains shown as sticks. d, Central region of SpdC between several transmembrane helices in the same orientation as shown in b.
Extended Data Fig. 8 The interface between the transmembrane helices of SagB and SpdC is crucial for complexation.
a, Cartoon representation of the SagB-SpdC complex shows that the transmembrane (TM) helix of SagB is making close contacts to the TM3 of SpdC. b, Close-up of the interface between the SagB TM helix and SpdC. Left panel: Surface representation, Right panel: the same view showing the side-chains of the SagB TM helix as sticks. c, An attempt to co-purify ΔTM-SagB with SpdC did not yield a complex, although the individual components could be recovered after their corresponding affinity purification steps, as shown in SDS-PAGE. d, Alignment of sequences corresponding to the TM helices of SagA and SagB shows significant sequence differences. A construct of SagB containing the TM helix of SagA was co-expressed with SpdC. A stable complex did not form between SpdC and SagB with the TM of SagA, and very little of this SagB variant co-purified along SpdC as shown in SDS-PAGE. Inversely, a construct of SagA containing the TM of SagB was co-expressed with SpdC and did not form a stable 1:1 complex (right gel). e, Radiolabeled peptidoglycan oligomers treated with SagB-SpdC, individual hydrolases, or hydrolases with the addition of SpdC. Unlike SagB, SagA activity is not significantly altered by the presence of SpdC. f, Swapping the TM of SagB onto SagA has only a minor effect on its product distribution.
Extended Data Fig. 9 Alignment of SpdC and Rce1 structures shows broad similarities in their transmembrane domains, but key differences in the predicted substrate binding site.
a, Cartoon representation of the transmembrane helices of SagB-SpdC (violet, green) aligned with the structure of Rce1 (blue, PDB# 4CAD), with arrows denoting the views in b. b, The aligned structures when viewed from within the plane of the membrane. Although the helices of Rce1 and SpdC are threaded in the same pattern, the helices differ in length and the loops between helices adopt significantly different conformations. c, Several Rce1 catalytic residues (blue sticks) are not present in SpdC (green sticks); conserved residues are labeled in black. d, Surface electrostatic maps reveal that Rce1 (left) and SpdC (right) both contain deep central cavities that open to the membrane (circled in black). Both Rce1 and SagB-SpdC are oriented as in a, showing that the cavities open to the membrane at different locations and that the rim of the SpdC cavity contains more positively charged residues.
Extended Data Fig. 10 Removal of the lipid carrier from nascent peptidoglycan inhibits SagB-SpdC cleavage.
a, Schematic of experiment shown in b. b, Radiolabeled peptidoglycan oligomers (lane 3) were treated with Colicin M (lane 4), which removes the lipid carrier, leaving a pyrophosphate on the reducing terminus of the glycan. Lipid-linked and de-lipidated oligomers were then treated with SagB-SpdC (lanes 5 and 6, respectively). Comparison of lane 3 to lane 5 shows changes to the sample characteristic of SagB-SpdC activity, whereas comparison of lane 4 to lane 6 shows no change, indicating that SagB-SpdC was not able to cleave oligomers lacking the lipid carrier.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15 and Tables 1–4, and the unmodified gels and autoradiographs.
Source data
Source Data Fig. 1
Unprocessed protein gels shown in Fig. 1.
Source Data Fig. 2
Unprocessed autoradiographs shown in Fig. 2.
Source Data Fig. 3
Unprocessed autoradiographs shown in Fig. 3.
Source Data Extended Data Fig. 1
Unprocessed autoradiograph shown in Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Unprocessed western blot shown in Extended Data Fig. 2.
Source Data Extended Data Fig. 4
Unmodified fluorescent gel shown in Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Unmodified autoradiograph shown in Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Unmodified western blot shown in Extended Data Fig. 6.
Source Data Extended Data Fig. 8
Unmodified protein gels and autoradiographs shown in Extended Data Fig. 8.
Source Data Extended Data Fig. 10
Unmodified autoradiograph shown in Extended Data Fig. 10.
Rights and permissions
About this article
Cite this article
Schaefer, K., Owens, T.W., Page, J.E. et al. Structure and reconstitution of a hydrolase complex that may release peptidoglycan from the membrane after polymerization. Nat Microbiol 6, 34–43 (2021). https://doi.org/10.1038/s41564-020-00808-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-020-00808-5
This article is cited by
-
FacZ is a GpsB-interacting protein that prevents aberrant division-site placement in Staphylococcus aureus
Nature Microbiology (2024)