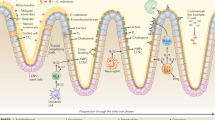
Abstract
Enteric pathogens sense the complex chemistry within the gastrointestinal tract to efficiently compete with the resident microbiota and establish a colonization niche. Here, we show that enterohaemorrhagic Escherichia coli and Citrobacter rodentium, its surrogate in a mouse infection model, sense galacturonic acid to initiate a multi-layered program towards successful mammalian infection. Galacturonic acid utilization as a carbon source aids the initial pathogen expansion. The main source of galacturonic acid is dietary pectin, which is converted to galacturonic acid by the prominent member of the microbiota, Bacteroides thetaiotamicron. This is regulated by the ExuR transcription factor. However, galacturonic acid is also sensed as a signal through ExuR to modulate the expression of the genes encoding a molecular syringe known as a type III secretion system, leading to infectious colitis and inflammation. Galacturonic acid acts as both a nutrient and a signal directing the exquisite microbiota–pathogen relationships within the gastrointestinal tract. This work highlights that differential dietary sugar availability influences the relationship between the microbiota and enteric pathogens, as well as disease outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. RNA-sequencing data can be accessed at European Nucleotide Archive under accession number PRJEB30676.
References
Sonnenburg, J. L. et al. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307, 1955–1959 (2005).
Baumler, A. J. & Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535, 85–93 (2016).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140 (2004).
Luperchio, S. A. & Schauer, D. B. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3, 333–340 (2001).
Kamada, N. et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329 (2012).
Slater, S. L., Sagfors, A. M., Pollard, D. J., Ruano-Gallego, D. & Frankel, G. The type III secretion system of pathogenic Escherichia coli. Curr. Top. Microbiol. Immunol. 416, 51–72 (2018).
Jarvis, K. G. et al. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc. Natl Acad. Sci. USA 92, 7996–8000 (1995).
McDaniel, T. K., Jarvis, K. G., Donnenberg, M. S. & Kaper, J. B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc. Natl Acad. Sci. USA 92, 1664–1668 (1995).
Chang, D.-E. et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl Acad. Sci. USA 101, 7427–7432 (2004).
Fabich, A. J. et al. Comparison of carbon nutrition for pathogenic and commensal Escherichia coli strains in the mouse intestine. Infect. Immun. 76, 1143–1152 (2008).
Maltby, R., Leatham-Jensen, M. P., Gibson, T., Cohen, P. S. & Conway, T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS ONE 8, e53957 (2013).
Peekhaus, N. & Conway, T. What's for dinner?: Entner–Doudoroff metabolism in Escherichia coli. J. Bacteriol. 180, 3495–3502 (1998).
Pifer, R., Russell, R. M., Kumar, A., Curtis, M. M. & Sperandio, V. Redox, amino acid, and fatty acid metabolism intersect with bacterial virulence in the gut. Proc. Natl Acad. Sci. USA 115, E10712–E10719 (2018).
Robert-Baudouy, J., Portalier, R. & Stoeber, F. Regulation of hexuronate system genes in Escherichia coli K-12: multiple regulation of the uxu operon by exuR and uxuR gene products. J. Bacteriol. 145, 211–220 (1981).
Blanco, C., Ritzenthaler, P. & Kolb, A. The regulatory region of the uxuAB operon in Escherichia coli K12. Mol. Gen. Genet. 202, 112–119 (1986).
Ritzenthaler, P., Blanco, C. & Mata-Gilsinger, M. Genetic analysis of uxuR and exuR genes: evidence for ExuR and UxuR monomer repressors interactions. Mol. Gen. Genet. 199, 507–511 (1985).
Rodionov, D. A., Mironov, A. A., Rakhmaninova, A. B. & Gelfand, M. S. Transcriptional regulation of transport and utilization systems for hexuronides, hexuronates and hexonates in gamma purple bacteria. Mol. Microbiol. 38, 673–683 (2000).
Suvorova, I. A. et al. Comparative genomic analysis of the hexuronate metabolism genes and their regulation in gammaproteobacteria. J. Bacteriol. 193, 3956–3963 (2011).
Tutukina, M. N., Potapova, A. V., Cole, J. A. & Ozoline, O. N. Control of hexuronate metabolism in Escherichia coli by the two interdependent regulators, ExuR and UxuR: derepression by heterodimer formation. Microbiology 162, 1220–1231 (2016).
Mellies, J. L., Elliott, S. J., Sperandio, V., Donnenberg, M. S. & Kaper, J. B. The Per regulon of enteropathogenic Escherichia coli: identification of a regulatory cascade and a novel transcriptional activator, the locus of enterocyte effacement (LEE)-encoded regulator (Ler). Mol. Microbiol. 33, 296–306 (1999).
Sperandio, V., Mellies, J. L., Nguyen, W., Shin, S. & Kaper, J. B. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA 96, 15196–15201 (1999).
Njoroge, J. W., Nguyen, Y., Curtis, M. M., Moreira, C. G. & Sperandio, V. Virulence meets metabolism: Cra and KdpE gene regulation in enterohemorrhagic Escherichia coli. mBio 3, e00280–00212 (2012).
Borenshtein, D., Nambiar, P. R., Groff, E. B., Fox, J. G. & Schauer, D. B. Development of fatal colitis in FVB mice infected with Citrobacter rodentium. Infect. Immun. 75, 3271–3281 (2007).
Winter, S. E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013).
Carlson-Banning, K. M. & Sperandio, V. Catabolite and oxygen regulation of enterohemorrhagic Escherichia coli virulence. mBio 7, e01852–16 (2016).
Mundy, R., MacDonald, T. T., Dougan, G., Frankel, G. & Wiles, S. Citrobacter rodentium of mice and man. Cell. Microbiol. 7, 1697–1706 (2005).
Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 (2008).
Luis, A. S. et al. Dietary pectic glycans are degraded by coordinated enzyme pathways in human colonic Bacteroides. Nat. Microbiol. 3, 210–219 (2018).
Ndeh, D. et al. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature 544, 65–70 (2017).
Curtis, M. M. et al. The gut commensal Bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe 16, 759–769 (2014).
Umar, S., Morris, A. P., Kourouma, F. & Sellin, J. H. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Prolif. 36, 361–375 (2003).
Kuss, S. K. et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252 (2011).
Sassone-Corsi, M. & Raffatellu, M. No vacancy: how beneficial microbes cooperate with immunity to provide colonization resistance to pathogens. J. Immunol. 194, 4081–4087 (2015).
Cameron, E. A. & Sperandio, V. Frenemies: signaling and nutritional integration in pathogen–microbiota–host interactions. Cell Host Microbe 18, 275–284 (2015).
Pacheco, A. R. & Sperandio, V. Enteric pathogens exploit the microbiota-generated nutritional environment of the gut. Microbiol. Spectr. 3, MBP-0001-2014 (2014).
Bohnhoff, M., Drake, B. L. & Miller, C. P. Effect of streptomycin on susceptibility of intestinal tract to experimental Salmonella infection. Proc. Soc. Exp. Biol. Med. 86, 132–137 (1954).
Pacheco, A. R. et al. Fucose sensing regulates bacterial intestinal colonization. Nature 492, 113–117 (2012).
Knutton, S. et al. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17, 2166–2176 (1998).
Kendall, M. M., Gruber, C. C., Parker, C. T. & Sperandio, V. Ethanolamine controls expression of genes encoding components involved in interkingdom signaling and virulence in enterohemorrhagic Escherichia coli O157:H7. mBio 3, e00050-12 (2012).
Gonyar, L. A. & Kendall, M. M. Ethanolamine and choline promote expression of putative and characterized fimbriae in enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 82, 193–201 (2014).
Porter, N. T., Luis, A. S. & Martens, E. C. Bacteroides thetaiotaomicron. Trends Microbiol. 26, 966–967 (2018).
Griffin, P. M. et al. Illnesses associated with Escherichia coli 0157:H7 infections. A broad clinical spectrum. Ann. Intern. Med. 109, 705–712 (1988).
Barthold, S. W., Coleman, G. L., Bhatt, P. N., Osbaldiston, G. W. & Jonas, A. M. The etiology of transmissible murine colonic hyperplasia. Lab. Anim. Sci. 26, 889–894 (1976).
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Knutton, S., Baldwin, T., Williams, P. H. & McNeish, A. S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 57, 1290–1298 (1989).
Acknowledgements
This study was supported by the National Institutes of Health grants AI053067, AI05135, AI077613 and AI114511 to V.S. A.G.J. was supported through National Institutes of Health Training Grant 5 T32 AI7520.
Author information
Authors and Affiliations
Contributions
A.G.J. conceived the studies, performed experiments and data analysis and wrote the paper. M.E. performed histological analysis and performed some mouse experiments. W.A. advised on experiments with Bt and pectin degradation. V.S. supervised all experiments, analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 ExuR regulates genes encoding enzymes necessary for the catabolism of galacturonic-acid.
a, Schematic representation of ExuR repression of the galacturonic-acid utilization genes. b, qRT-PCR of the galacturonic utilization genes in WT (n = 3) and ΔexuR (n = 3) EHEC (n, number of biological replicates; results are representative of two independent experiments). The mean and P value for two-sided Mann-Whitney statistical analysis are shown. c, Competition EMSA of ExuR binding to the ler promoter in the absence and presence of ler cold probe. Results are representative of two independent experiments with similar results. d, qRT-PCR of the LEE espA gene in EPEC in the presence of vehicle (n = 6) or galacturonic-acid (GalA) (n = 6) (n, number of biological replicates; results are representative of two independent experiments). The mean and P value for two-sided Mann-Whitney statistical analysis are shown.
Extended Data Fig. 2 UxaC and galacturonic acid promotion of EHEC’s growth.
a, Growth curves of WT (n = 2) and ΔuxaC (n = 2) EHEC in M9 minimal medium with 5mM galacturonic-acid as a sole carbon source (n, number of biological replicates; results are representative of one independent experiments). The mean ± SD and P value for two-sided two-way ANOVA statistical analysis are shown. b, Growth curves of WT (n = 4) and ΔuxaC (n = 4) EHEC in low-glucose DMEM (n, number of biological replicates; results are representative of one independent experiments). The mean and P value for two-sided one-way ANOVA statistical analysis are shown. c, Growth of WT EHEC in low-glucose DMEM supplemented with different concentrations of galacturonic acid.
Extended Data Fig. 3 ExuR also activates the LEE in C. rodentium.
a, Growth curve of WT (n = 3) and ΔexuR (n = 3) C. rodentium strains grown in low-glucose DMEM under microaerobic conditions (n, number of biological replicates; results are representative of one independent experiments). The mean ± SD value for two-sided one-way ANOVA statistical analysis are shown. b, RT-qPCR of the LEE-encoded genes in escC, escV, tir, and espA in WT (n = 9) and ΔexuR (n = 9) C. rodentium (n, number of biological replicates; results are representative of three independent experiments). The mean and P value for two-sided two-way ANOVA statistical analysis are shown. c, Western blot for secreted EspB in WT and ΔexuR C. rodentium. Representative blots from three independent experiments. d, Growth curves of C. rodentium with glucose (Glu) (n = 3), galacturonic acid (GalA) (n = 3) or glucuronic acid (GlcA) (n = 3) as sole carbon sources (n, number of biological replicates; results are representative of three independent experiments). The mean ± SD value for two-sided one-way ANOVA statistical analysis are shown.
Extended Data Fig. 4 Galacturonic-acid acts through ExuR to decrease LEE gene expression.
Western blot of supernatants from EHEC WT, ΔexuR, ΔuxuR and ΔexuRuxuR (ΔΔ) grown in the presence of glucose (Glu), galacturonic-acid (GalA), or glucuronic acid (GlcA) in DMEM probed with anti-EspA antiserum. BSA is used as a loading control. Representative blots from three independent experiments.
Extended Data Fig. 5 Mice infected with ΔuxaC C. rodentium have only a mild increase in inflammation.
C3H/HeJ mice under non-infected conditions as well as at post-infection day 8 with WT or the ΔexuR, ΔuxaC and ΔexuRΔuxaC mutants. a, Haematoxylin-eosin-stained cecal patch tissues of C3H/HeJ mice. Representative images from two independent experiments, scale bars = 100 µm. b, Blinded histopathology scores of non-infected mice or infected with WT (n = 4) or the ΔexuR (n = 4), ΔuxaC (n = 4), and ΔexuRΔuxaC (n = 4) C. rodentium (n, number of biological replicates; results are representative of two independent experiments). The mean and P value for two-sided one-way ANOVA statistical analysis.
Extended Data Fig. 6 Pectin engenders inflammation in C3H/HeJ mice.
C3H/HeJ mice were treated with 200µL of 2% pectin or PBS. a, Blinded histopathology scores of non-infected mice treated with 200µL of 2% pectin (n = 4) or PBS (n = 4) (n, number of biological replicates; results are representative of two independent experiments). The mean and P value for two-sided one-way ANOVA statistical analysis. b-c, RT-qPCR determined the expression of Nos2 and IL22 genes in the cecal tissue of mice treated with 200µL of 2% pectin (n = 4) or PBS (n = 4) (n, number of biological replicates; results are representative of two independent experiments). The mean and P value for two-sided Mann-Whitney statistical analysis are shown. GAPDH was used to normalize gene expression.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 1
Statistical Source Data.
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical Source Data.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 2
Statistical Source Data.
Source Data Extended Data Fig. 3
Statistical Source Data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Jimenez, A.G., Ellermann, M., Abbott, W. et al. Diet-derived galacturonic acid regulates virulence and intestinal colonization in enterohaemorrhagic Escherichia coli and Citrobacter rodentium. Nat Microbiol 5, 368–378 (2020). https://doi.org/10.1038/s41564-019-0641-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0641-0
This article is cited by
-
Microbiota-mediated colonization resistance: mechanisms and regulation
Nature Reviews Microbiology (2023)
-
Gut Microbiota Resilience Mechanisms Against Pathogen Infection and its Role in Inflammatory Bowel Disease
Current Clinical Microbiology Reports (2023)