Abstract
Staphylococcus aureus small colony variants (SCVs) are frequently associated with chronic infection, yet they lack expression of many virulence determinants associated with the pathogenicity of wild-type strains. We found that both wild-type S. aureus and a ΔhemB SCV prototype potently activate glycolysis in host cells. Glycolysis and the generation of mitochondrial reactive oxygen species were sufficient to induce necroptosis, a caspase-independent mechanism of host cell death that failed to eradicate S. aureus and instead promoted ΔhemB SCV pathogenicity. To support ongoing glycolytic activity, the ΔhemB SCV induced over a 100-fold increase in the expression of fumC, which encodes an enzyme that catalyses the degradatin of fumarate, an inhibitor of glycolysis. Consistent with fumC-dependent depletion of local fumarate, the ΔhemB SCV failed to elicit trained immunity and protection from a secondary infectious challenge in the skin. The reliance of the S. aureus SCV population on glycolysis accounts for much of its role in the pathogenesis of S. aureus skin infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Whole genome sequence data of the ΔhemB, ΔfumC and ΔhemBΔfumC LAC mutants and the complemented strains ΔhemB (hemB) and ΔfumC (fumC) were submitted to the European Nucleotide Archive under BioProject number PRJEB30093, accession numbers ERS3011129, ERS3011131, ERS3398722, ERS3011130 and ERS3011132, respectively. Data that support the findings of this study are available from the corresponding author upon request.
References
Kahl, B. C., Becker, K. & Loffler, B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 29, 401–427 (2016).
Kennedy, A. D. et al. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J. Infect. Dis. 202, 1050–1058 (2010).
Kernodle, D. S., Voladri, R. K., Menzies, B. E., Hager, C. C. & Edwards, K. M. Expression of an antisense hla fragment in Staphylococcus aureus reduces alpha-toxin production in vitro and attenuates lethal activity in a murine model. Infect. Immun. 65, 179–184 (1997).
Kobayashi, S. D. et al. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J. Infect. Dis. 204, 937–941 (2011).
Menzies, B. E. & Kernodle, D. S. Site-directed mutagenesis of the alpha-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62, 1843–1847 (1994).
Proctor, R. A. & Peters, G. Small colony variants in staphylococcal infections: diagnostic and therapeutic implications. Clin. Infect. Dis. 27, 419–422 (1998).
Proctor, R. A. et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 4, 295–305 (2006).
Kriegeskorte, A. et al. Staphylococcus aureus small colony variants show common metabolic features in central metabolism irrespective of the underlying auxotrophism. Front. Cell. Infect. Microbiol. 4, 141 (2014).
Proctor, R. A. et al. Staphylococcus aureus small colony variants (SCVs): a road map for the metabolic pathways involved in persistent infections. Front. Cell. Infect. Microbiol. 4, 99 (2014).
Tuchscherr, L. et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J. Infect. Dis. 202, 1031–1040 (2010).
Arts, R. J., Joosten, L. A. & Netea, M. G. Immunometabolic circuits in trained immunity. Semin. Immunol. 28, 425–430 (2016).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Wickersham, M. et al. Metabolic stress drives keratinocyte defenses against Staphylococcus aureus infection. Cell Rep. 18, 2742–2751 (2017).
Kitur, K. et al. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 16, 2219–2230 (2016).
Baek, K. T. et al. Genetic variation in the Staphylococcus aureus 8325 strain lineage revealed by whole-genome sequencing. PLoS ONE 8, e77122 (2013).
Herbert, S. et al. Repair of global regulators in Staphylococcus aureus 8325 and comparative analysis with other clinical isolates. Infect. Immun. 78, 2877–2889 (2010).
Horsburgh, M. J. et al. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184, 5457–5467 (2002).
Giachino, P., Engelmann, S. & Bischoff, M. ςB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183, 1843–1852 (2001).
Anzaldi, L. L. & Skaar, E. P. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 78, 4977–4989 (2010).
Atalla, H., Gyles, C. & Mallard, B. Staphylococcus aureus small colony variants (SCVs) and their role in disease. Anim. Health Res. Rev. 12, 33–45 (2011).
von Eiff, C. et al. A site-directed Staphylococcus aureus hemB mutant is a small-colony variant which persists intracellularly. J. Bacteriol. 179, 4706–4712 (1997).
Palma Medina, L. M. et al. Metabolic cross-talk between human bronchial epithelial cells and internalized Staphylococcus aureus as a driver for infection. Mol. Cell. Proteom. 18, 892–908 (2019).
LaRocca, T. J., Sosunov, S. A., Shakerley, N. L., Ten, V. S. & Ratner, A. J. Hyperglycemic conditions prime cells for RIP1-dependent Necroptosis. J. Biol. Chem. 291, 13753–13761 (2016).
McCaig, W. D. et al. Hyperglycemia potentiates a shift from apoptosis to RIP1-dependent necroptosis. Cell Death Discov. 4, 55 (2018).
Cai, Z. et al. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 16, 55–65 (2014).
Chen, X. et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 24, 105–121 (2014).
Gonzalez-Juarbe, N. et al. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog. 11, e1005337 (2015).
Kitur, K. et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 11, e1004820 (2015).
Vitko, N. P., Spahich, N. A. & Richardson, A. R. Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. mBio 6, e00045–15 (2015).
Yang, Z. et al. RIP3 targets pyruvate dehydrogenase complex to increase aerobic respiration in TNF-induced necroptosis. Nat. Cell Biol. 20, 186–197 (2018).
Kucera, O. et al. The effect of tert-butyl hydroperoxide-induced oxidative stress on lean and steatotic rat hepatocytes in vitro. Oxid. Med. Cell. Longev. 2014, 752506 (2014).
Galluzzi, L., Kepp, O., Chan, F. K. & Kroemer, G. Necroptosis: mechanisms and relevance to disease. Annu. Rev. Pathol. 12, 103–130 (2017).
Goodall, M. L. et al. The autophagy machinery controls cell death switching between apoptosis and necroptosis. Dev. Cell 37, 337–349 (2016).
Bhattacharyya, S. et al. Small colony variants of Staphylococcus aureus isolated from a patient with infective endocarditis: a case report and review of the literature. Iran. J. Microbiol. 4, 98–99 (2012).
Jonsson, I. M. et al. Virulence of a hemB mutant displaying the phenotype of a Staphylococcus aureus small colony variant in a murine model of septic arthritis. Microb. Pathog. 34, 73–79 (2003).
Chan, L. C. et al. Innate immune memory contributes to host defense against recurrent skin and skin structure infections caused by methicillin-resistant staphylococcus aureus. Infect. Immun. 85, e00876–16 (2017).
Chan, L. C. et al. Protective immunity in recurrent Staphylococcus aureus infection reflects localized immune signatures and macrophage-conferred memory. Proc. Natl Acad. Sci. USA 115, E11111–E11119 (2018).
van der Heijden, C. et al. Epigenetics and trained immunity. Antioxid. Redox Signal. 29, 1023–1040 (2018).
Arts, R. J. et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 24, 807–819 (2016).
Kornberg, M. D. et al. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 360, 449–453 (2018).
Naik, S. et al. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480 (2017); erratum 560, E2 (2018).
Monk, I. R., Tree, J. J., Howden, B. P., Stinear, T. P. & Foster, T. J. Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6, e00308–e00315 (2015).
Monk, I. R., Howden, B. P., Seemann, T. & Stinear, T. P. Correspondence: Spontaneous secondary mutations confound analysis of the essential two-component system WalKR in Staphylococcus aureus. Nat. Commun. 8, 14403 (2017).
Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 111, A3.B.1–A3.B.3 (2015).
Melamud, E., Vastag, L. & Rabinowitz, J. D. Metabolomic analysis and visualization engine for LC-MS data. Anal. Chem. 82, 9818–9826 (2010).
Barker, M. & Rayens, W. Partial least squares for discrimination. J. Chemom. 17, 166–173 (2003).
Rohart, F., Gautier, B., Singh, A. & Le Cao, K. A. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13, e1005752 (2017).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Berger, S. B. et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 192, 5476–5480 (2014).
Soong, G. et al. Methicillin-resistant Staphylococcus aureus adaptation to human keratinocytes. mBio 6, e00289–15 (2015).
Acknowledgements
We thank I. Lewis (University of Calgary), R. Groves (University of Calgary) and D. P. De Souza (Metabolomics Australia) for their support with the metabolomics studies and V. J. Torres (New York University School of Medicine) for his gift of antibodies against staphylococci toxins. This work was supported by NIH grant R01AI103854 to A.P., NIH grant S10RR027050 to the Columbia Center for Translational Immunology Flow Cytometry Core and NHMRC grant (Australia) APP1066791 to B.P.H.
Author information
Authors and Affiliations
Contributions
T.W.F.L. and A.P. conceived the project, designed the experiments and interpreted the data. T.W.F.L. performed the in vitro and in vivo experiments and data analysis. I.R.M. constructed the bacterial mutants and contributed to the transcriptomics studies. K.P.A. contributed to the in vitro and in vivo work pertaining to Aim2. N.W. performed the in vitro THP-1 infection and A.M. analysed the metabolomics data. S.A.R., S.P., L.P.N., F.D. and S.J.G. assisted with the in vitro and in vivo experiments and data analysis. T.W.F.L., B.P.H. and A.P. wrote and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
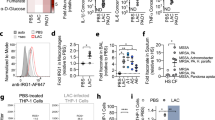
Extended data 1 ΔhemB LAC SCVs stimulate a distinct metabolic response in THP-1 cells.
(A) Construction of the ΔhemB mutant in the USA300 LAC background. (B) White light image of WT LAC and the ΔhemB LAC mutant on BHI and blood agar plates to better illustrate pinpoint size of the ΔhemB LAC mutant on BHI agar and β (complete)-hemolysis on blood agar, image shown is from 1 representative experiment, n = 3 independent experiments. (C) Representative PCA score plots showing principal component 1 (PC1/variate 1) versus PC2/variate 2 of the total intracellular and extracellular THP-1 metabolomes, n = 2 independent experiments in triplicate. (D) Heatmaps of the total intracellular and extracellular THP-1 metabolomes, n = 2. (E) Heatmap of lower abundance metabolites in the extracellular THP-1 metabolomes, n = 2. (F) Relative quantification of fumarate in the extracellular metabolome of uninfected and infected THP-1 cells. Data represent median with 95% CI, n = 2 independent experiments in triplicate.
Extended data 2 Necroptosis promotes ΔhemB SCV persistence.
(A) A representative immunoblot showing the induction of PMLKL and PS6 in HEKn cells exposed to PBS, WT LAC, ΔhemB LAC or the complemented strain for 4 h in the presence and absence of 2-DG or BHA, n = 3 independent experiments. (B) Bacterial load in skin biopsies 5 days following infection of WT C57BL/6 or Ripk1d or Mlkl−/− mice with WT 8325-4 and ΔhemB 8325-4. (C) Innate immune cell recruitment from (B) at day 5. (D) Cytokine measurements from mouse skin biopsies from (B) at day 5 For (B-D), data represent mean ± SEM, n = 15 (C57BL/6 WT), n = 17 (C57BL/6 ΔhemB). n = 15 (Ripk1d WT), n = 12 (Ripk1d ΔhemB), n = 9 (Mlkl−/− WT) or n = 10 (Mlkl−/− ΔhemB) independent animals. Statistical analysis was performed by one-way ANOVA, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
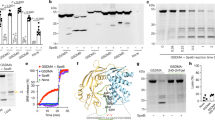
Extended data 3 Characterization of the ΔhemBΔfumC double mutant.
(A) Growth of the ΔhemBΔfumC double mutant as compared to WT LAC, the ΔhemB or ΔfumC single mutants on BHI and blood agar plates. Various dilutions of bacterial cultures were plated. Image shown is from 1 representative experiment, n = 3 independent experiments. (B) Growth of the ΔhemBΔfumC double mutant as compared to WT LAC and the ΔhemB single mutant in liquid LB culture. n = 3 independent experiments. (C) Cytokine measurements from the skin of mice 5 days post infection with WT LAC or the ΔhemBΔfumC mutant. Data represent mean ± SEM, n = 4 independent animal samples.. Statistical analysis was performed by one-way ANOVA, ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05.
Extended data 4 Protection against secondary S. aureus infection is independent of IL1R1-Aim2 activation.
(A-B) (A) Dermonecrosis and (B) bacterial load in WT C57BL/6 and Il1r1−/− mice treated with PBS or infected with WT LAC on day 0 and challenged at the same site on day 28 with WT LAC; black asterisks denote statistical differences in lesion size with respect to uninfected mice, purple asterisks show statistical difference between WT C57BL/6 and Il1r1−/−-infected mice and green asterisks show statistical differences between WT C57BL/6 and Il1r1−/−-naïve mice upon secondary challenge, n = 2 (C57BL/6 or Il1r1−/− PBS PBS), n = 5 (C57BL/6 PBS WT or C57BL/6 WT WT), n = 4 (Il1r1−/− PBS WT) or n = 6 (Il1r1−/− WT WT) independent animals. (C-D) (C) Dermonecrosis and (D) bacterial load in the skin of WT C57BL/6 and Aim2−/− mice 5 days post infection with WT LAC, ns: not significant, n = 3 or 4 independent animals (day 1) or n = 10 independent animals (day 5). (E) Intracellular mean fluorescence intensity (MFI) of Aim2 measured by flow cytometry in keratinocytes infected with WT LAC or transfected with poly dA:dT for 24 h, n = 1 representative experiment from 3 independent experiments. (F) Expression of Aim2 in HEKn cells infected with WT LAC or transfected with poly dA:dT for 24 h, compared to uninfected cells (PBS) by qRT-PCR, n = 1 representative experiment from 3 independent experiments, For (A-F), data represent mean ± SEM. Statistical analysis was performed by one-way (B) or two-way ANOVA (A, C and D), ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns; p = 0.9999 (C, day 1), 0.9051 (C, day 2), 0.6533 (C, day 3), 0.9820 (C, day 4), 0.9899 (C, day 5) and p = 0.0892 (D, day 1) and 0.4037 (D, day 5).
Supplementary information
Supplementary Tables 1–6
Supplementary Table 1: bacterial strains used in this study. Supplementary Table 2: primers used in this study. Supplementary Table 3: plasmids used in this study. Supplementary Table 4: list of extracellular metabolites from infected human PBMCs. Supplementary Table 5: list of intracellular metabolites from infected THP-1 cells. Supplementary Table 6: list of extracellular metabolites from infected THP-1 cells.
Source data
Source Data Fig. 1
Full blot.
Source Data Fig. 2
Full blot.
Source Data Fig. 3
Full blot.
Source Data Extended Data Fig. 1
Full blot.
Source Data Extended Data Fig. 2
White light image of agar plates.
Source Data Extended Data Fig. 3
Picture of agar plates.
Rights and permissions
About this article
Cite this article
Wong Fok Lung, T., Monk, I.R., Acker, K.P. et al. Staphylococcus aureus small colony variants impair host immunity by activating host cell glycolysis and inducing necroptosis. Nat Microbiol 5, 141–153 (2020). https://doi.org/10.1038/s41564-019-0597-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0597-0
This article is cited by
-
Staphylococcus aureus host interactions and adaptation
Nature Reviews Microbiology (2023)
-
The double-edged functions of necroptosis
Cell Death & Disease (2023)
-
Necroptosis-mediated HMGB1 secretion of keratinocytes as a key step for inflammation development in contact hypersensitivity
Cell Death Discovery (2022)
-
Staphylococcal superantigen-like protein 10 induces necroptosis through TNFR1 activation of RIPK3-dependent signal pathways
Communications Biology (2022)
-
Extracellular vesicles, a novel model linking bacteria to ferroptosis in the future?
Applied Microbiology and Biotechnology (2022)