Abstract
Innate immunity is the first line of host defence against pathogens. Suppression of innate immune responses is essential for the survival of all viruses. However, the interplay between innate immunity and HIV/SIV is only poorly characterized. We have discovered Vpx as a novel inhibitor of innate immune activation that associates with STING signalosomes and interferes with the nuclear translocation of NF-κB and the induction of innate immune genes. This new function of Vpx could be separated from its role in mediating degradation of the antiviral factor SAMHD1, and is conserved among diverse HIV-2/SIV Vpx. Vpx selectively suppressed cGAS–STING-mediated nuclear factor-κB signalling. Furthermore, Vpx and Vpr had complementary activities against cGAS–STING activity. Since SIVMAC lacking both Vpx and Vpr was less pathogenic than SIV deficient for Vpr or Vpx alone, suppression of innate immunity by HIV/SIV is probably a key pathogenic determinant, making it a promising target for intervention.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Microarray data have been deposited into the National Center for Biotechnology Information GEO public database under the accession code GSE117984. Raw data from all of the other figures, and unique materials, including viruses and plasmids, are available from the corresponding author on request.
References
Henderson, L. E., Sowder, R. C., Copeland, T. D., Benveniste, R. E. & Oroszlan, S. Isolation and characterization of a novel protein (X-ORF product) from SIV and HIV-2. Science 241, 199–201 (1988).
Yu, X. F., Ito, S., Essex, M. & Lee, T. H. A naturally immunogenic virion-associated protein specific for HIV-2 and SIV. Nature 335, 262–265 (1988).
Yu, X. F., Yu, Q. C., Essex, M. & Lee, T. H. The vpx gene of simian immunodeficiency virus facilitates efficient viral replication in fresh lymphocytes and macrophage. J. Virol. 65, 5088–5091 (1991).
Goujon, C. et al. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 4, 2 (2007).
Mangeot, P. et al. High levels of transduction of human dendritic cells with optimized SIV vectors. Mol. Ther. 5, 283–290 (2002).
Fujita, M. et al. Vpx is critical for reverse transcription of the human immunodeficiency virus type 2 genome in macrophages. J. Virol. 82, 7752–7756 (2008).
Sharova, N. et al. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 4, e1000057 (2008).
Srivastava, S. et al. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 4, e1000059 (2008).
Hrecka, K. et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 474, 658–661 (2011).
Laguette, N. et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474, 654–657 (2011).
Goldstone, D. C. et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 480, 379–382 (2011).
Ji, X. et al. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat. Struct. Mol. Biol. 20, 1304–1309 (2013).
Powell, R. D., Holland, P. J., Hollis, T. & Perrino, F. W. Aicardi–Goutières syndrome gene and HIV-1 restriction factor SAMHD1 is a dGTP-regulated deoxynucleotide triphosphohydrolase. J. Biol. Chem. 286, 43596–43600 (2011).
Ryoo, J. et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat. Med. 20, 936–941 (2014).
Yan, J. et al. Tetramerization of SAMHD1 is required for biological activity and inhibition of HIV infection. J. Biol. Chem. 288, 10406–10417 (2013).
Zhu, C. et al. Structural insight into dGTP-dependent activation of tetrameric SAMHD1 deoxynucleoside triphosphate triphosphohydrolase. Nat. Commun. 4, 2722 (2013).
Lahouassa, H. et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 13, 223–228 (2012).
Shingai, M. et al. The expression of functional Vpx during pathogenic SIVMAC infections of rhesus macaques suppresses SAMHD1 in CD4+ memory T cells. PLoS Pathog. 11, e1004928 (2015).
Rice, G. et al. Mutations involved in Aicardi–Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 41, 829–832 (2009).
Clifford, R. et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood 123, 1021–1031 (2014).
Rentoft, M. et al. Heterozygous colon cancer-associated mutations of SAMHD1 have functional significance. Proc. Natl Acad. Sci. USA 113, 4723–4728 (2016).
Zhao, K. et al. Modulation of LINE-1 and Alu/SVA retrotransposition by Aicardi–Goutieres syndrome-related SAMHD1. Cell Rep. 4, 1108–1115 (2013).
Gramberg, T. et al. Restriction of diverse retroviruses by SAMHD1. Retrovirology 10, 26 (2013).
Coquel, F. et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature 557, 57–61 (2018).
Daddacha, W. et al. SAMHD1 promotes DNA end resection to facilitate DNA repair by homologous recombination. Cell Rep. 20, 1921–1935 (2017).
Chen, S. et al. SAMHD1 suppresses innate immune responses to viral infections and inflammatory stimuli by inhibiting the NF-κB and interferon pathways. Proc. Natl Acad. Sci. USA 115, E3789–E3807 (2018).
Compton, A. A. et al. IFITM proteins incorporated into HIV-1 virions impair viral fusion and spread. Cell Host Microbe 16, 736–747 (2014).
Goujon, C. et al. Human MX2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature 502, 559–562 (2013).
Kane, M. et al. MX2 is an interferon-induced inhibitor of HIV-1 infection. Nature 502, 563–566 (2013).
Krapp, C. et al. Guanylate binding protein (GBP) 5 is an interferon-inducible inhibitor of HIV-1 infectivity. Cell Host Microbe 19, 504–514 (2016).
Liu, Z. et al. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell Host Microbe 14, 398–410 (2013).
Ma, Z. et al. Modulation of the cGAS–STING DNA sensing pathway by gammaherpesviruses. Proc. Natl Acad. Sci. USA 112, 4306–4315 (2015).
Ablasser, A. et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384 (2013).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Christensen, M. H. et al. HSV-1 ICP27 targets the TBK1-activated STING signalsome to inhibit virus-induced type I IFN expression. EMBO J. 35, 1385–1399 (2016).
Lau, L., Gray, E. E., Brunette, R. L. & Stetson, D. B. DNA tumor virus oncogenes antagonize the cGAS–STING DNA-sensing pathway. Science 350, 568–571 (2015).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009).
Zhang, J. et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe 24, 234–248 (2018).
Fu, Y. Z. et al. Human cytomegalovirus tegument protein UL82 inhibits STING-mediated signaling to evade antiviral immunity. Cell Host Microbe 21, 231–243 (2017).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Konno, H., Konno, K. & Barber, G. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 155, 688–698 (2013).
Xia, T., Konno, H., Ahn, J. & Barber, G. N. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell Rep. 14, 282–297 (2016).
Hofmann, H. et al. The Vpx lentiviral accessory protein targets SAMHD1 for degradation in the nucleus. J. Virol. 86, 12552–12560 (2012).
Ahn, J. et al. HIV/simian immunodeficiency virus (SIV) accessory virulence factor Vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. J. Biol. Chem. 287, 12550–12558 (2012).
Schwefel, D. et al. Structural basis of lentiviral subversion of a cellular protein degradation pathway. Nature 505, 234–238 (2014).
Fregoso, O. I. et al. Evolutionary toggling of Vpx/Vpr specificity results in divergent recognition of the restriction factor SAMHD1. PLoS Pathog. 9, e1003496 (2013).
Lim, E. S. et al. The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell Host Microbe 11, 194–204 (2012).
Wang, H. et al. Inhibition of Vpx-mediated SAMHD1 and Vpr-mediated HLTF degradation by selective disruption of viral CRL4 (DCAF1) E3 ubiquitin ligase assembly. J. Virol. 91, e00225-17 (2017).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015).
Aroh, C. et al. Innate immune activation by cGMP-AMP nanoparticles leads to potent and long-acting antiretroviral response against HIV-1. J. Immunol. 199, 3840–3848 (2017).
Guyader, M., Emerman, M., Montagnier, L. & Peden, K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 8, 1169–1175 (1989).
Kappes, J. C., Conway, J. A., Lee, S. W., Shaw, G. M. & Hahn, B. H. Human immunodeficiency virus type 2 vpx protein augments viral infectivity. Virology 184, 197–209 (1991).
Tristem, M., Marshall, C., Karpas, A. & Hill, F. Evolution of the primate lentiviruses: evidence from vpx and vpr. EMBO J. 11, 3405–3412 (1992).
Chougui, G. et al. HIV-2/SIV viral protein X counteracts HUSH repressor complex. Nat. Microbiol. 3, 891–897 (2018).
Yurkovetskiy, L. et al. Primate immunodeficiency virus Vpx and Vpr counteract transcriptional repression of proviruses by the HUSH complex. Nat. Microbiol. 3, 1354–1361 (2018).
Baldauf, H. M. et al. Vpx overcomes a SAMHD1-independent block to HIV reverse transcription that is specific to resting CD4 T cells. Proc. Natl Acad. Sci. USA 114, 2729–2734 (2017).
Reinhard, C., Bottinelli, D., Kim, B. & Luban, J. Vpx rescue of HIV-1 from the antiviral state in mature dendritic cells is independent of the intracellular deoxynucleotide concentration. Retrovirology 11, 12 (2014).
Descours, B. et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4+ T-cells. Retrovirology 9, 87 (2012).
Bridgeman, A. et al. Viruses transfer the antiviral second messenger cGAMP between cells. Science 349, 1228–1232 (2015).
Gentili, M. et al. Transmission of innate immune signaling by packaging of cGAMP in viral particles. Science 349, 1232–1236 (2015).
Ablasser, A. et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503, 530–534 (2013).
Gibbs, J. S. et al. Progression to AIDS in the absence of a gene for vpr or vpx. J. Virol. 69, 2378–2383 (1995).
Westmoreland, S. V. et al. SIV vpx is essential for macrophage infection but not for development of AIDS. PLoS ONE 9, e84463 (2013).
Ferdinand, R. et al. Vpr enhances tumor necrosis factor production by HIV-1-infected T cells. J. Virol. 89, 12118–12130 (2015).
Trotard, M. et al. Sensing of HIV-1 infection in Tzm-bl cells with reconstituted expression of STING. J. Virol. 90, 2064–2076 (2016).
Laguette, N. et al. Premature activation of the SLX4 complex by Vpr promotes G2/M arrest and escape from innate immune sensing. Cell 156, 134–145 (2014).
Hotter, D. et al. Primate lentiviruses use at least three alternative strategies to suppress NF-κB-mediated immune activation. PLoS Pathog. 13, e1006598 (2017).
Rui, Y. et al. Disruption of MDA5-mediated innate immune responses by the 3C proteins of coxsackievirus A16, coxsackievirus A6, and enterovirus D68. J. Virol. 91, e00546-17 (2017).
Acknowledgements
This work was supported in part by funding from the Chinese Ministry of Science and Technology (number 2018ZX10731-101-001-014), National Natural Science Foundation of China (numbers 81772169, 81802351, 31900133 and 31970151), China Postdoctoral Science Foundation (numbers 2017M621912 and 2018M632450) and Advanced Postdoctoral Program of Zhejiang Province. We thank D. McClellan for editorial assistance, Q. Dong for confocal microscopy analysis, and M. Wu for technical assistance.
Author information
Authors and Affiliations
Contributions
J.S. and Y.R. carried out most of the biochemical experiments, with help from M.L., L.Y., Z.Zhou and S.S. M.L. and T.C. conducted the immunostaining and confocal microscopy experiments. H.X. performed the microarray data analysis. Z.Zhang and N.Z. contributed the key reagents and flow cytometry experiments. W.Z., Y.C. and R.M. performed the viral infection experiments and plasmid construction. S.Z., R.X. and W.W. contributed to supervision and data analysis. X.-F.Y. directed the project, analysed the data and wrote the paper, with help from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 SIVmac infection- mediated SAMHD1 depletion does not induce immune activation in THP-1, EA.hy926, HEK293T and HeLa cells.
A, Detection of endogenous STING and cGAS expression levels in THP-1, U937, HFF, and EA.hy926 cells (n = 2 independent biological experiments). B, THP-1 cells were treated with PMA (100 nM) for 4h and subsequently treated with 2’3’-cGAMP (4 μg/mL) for 12h. Total RNA was prepared and analyzed for the transcriptional level of the indicated genes by RT-qPCR (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. C,D, THP-1 cells were infected with an equal amount of SIVmac or SIVmacΔVpx virus for 12h. Cell lysates were analyzed by immunoblotting using SAMHD1 and CAp27 antibodies (C) (n = 3 independent biological experiments). Total RNA was prepared and analyzed for the indicated genes by RT-qPCR (D) (n = 3 independent biological experiments). Means and standard deviations are presented (B, D). The statistical significance analyses were performed using a two-sided unpaired t-test. E, EA.hy926 cells were transfected with ISD (2 μg/mL) for 12h, and total RNA was prepared and analyzed for the indicated genes by RT-qPCR (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. F,G, EA.hy926 cells were infected with an equal amount of SIVmac or SIVmacΔVpx virus for 12h. RT- qPCR (F) and immunoblotting (G) were performed as described in C,D (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. H, Endogenous SAMHD1 and p-SAMHD1 protein expression levels in THP-1, U937, and EA.hy926 cells (One representative immunoblotting result out of n = 2 independent biological experiments is shown.). I,K, HEK293T cells or J,L, HeLa cells were infected with an equal amount of SIVmac or SIVmacΔVpx virus for 12h. Immunoblotting and RT- qPCR were performed as described in C,D (n = 3 independent biological experiments). Means and standard deviations are presented (K, L). The statistical significance analyses were performed using a two-sided unpaired t-test. M, HEK293T, HeLa, EA.hy926, THP-1, CD4+ T cells, and MDM cells were infected with equal amounts of SIVΔEnv GFP or SIVΔEnvΔVpx GFP virus for 48h. The infected cells were then harvested, and GFP-positive cells were tested by flow cytometry (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using two-sided unpaired t-test.
Extended Data Fig. 2 Selective inhibition of cGAS-STING- but not RIG-I card- or IRF3-5D-triggered immune activation by SIVmac.
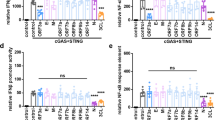
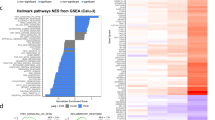
A,Vpx has no effect on cGAS or STING expression. Expression vectors for Myc-cGAS and STING-Flag were co-transfected with increasing amounts of SIVmac Vpx in HEK293T cells. After 24h, cell lysates were detected by immunoblotting with Myc, HA, and Flag antibodies (A representative immunoblotting result out of n = 3 independent biological experiments is shown.). B,C,D, HEK293T cells were transfected with NF-κB- Luc (B,C) or IRF3-Luc (D) with increasing amounts of SIVmac HA-Vpx, in the presence of RIG-IN-Flag (B), Myc-cGAS, and STING-Flag expression vectors (C), or IRF5-5D-Flag vector (D), respectively. pRL-TK Renilla was used as the internal control. Transactivation of the luciferase reporter was determined 24h after transfection, and the protein expression levels were analyzed by immunoblotting with anti-Flag or anti-HA antibody (n = 3 independent biological experiments). GAPDH served as a loading control. Means and standard deviations are presented; a two-sided paired t-test was performed. E, CD4+ T cells were infected with equal amounts of SIVmacΔVpx, SIVmacΔVpx+Vpx, or WT virus for 4h, the medium was changed, and SeV (20 HA/mL) was added to the medium and incubated for another 8 h. Total RNA was prepared from the harvested cells and analyzed for the transcriptional level of the indicated genes by RT-qPCR. Different shapes represent different donors, with three independent experiments for each donor. A single mean value, depicted as a single horizontal line, is shown and marked in red. Standard deviations are presented. (n = 3 independent biological experiments.) F, cGAS-STING- stimulated genes and the influence of Vpx. Heat map of the percentages of genes stimulated by cGAS+STING (FC>2) and regulated by Vpx .G, The numbers in the pie charts indicate the percentage (3.2%) of NF-κB-regulated genes (29) among the total cGAS-STING-stimulated genes (906) (FC>1.5) (left) and the percentage (90%) of NF-κB-regulated genes (29) suppressed by Vpx (26) (right) from microarray data. NF-κB- regulated genes are shown in purple, and others in dark yellow; NF-κB-regulated genes suppressed by Vpx are in red, and others in blue.
Extended Data Fig. 3 HIV-2/SIVmac Vpx suppresses NF-κB regulated genes in HEK293T cells and ISD- stimulated innate immune responses in EA.hy926 cells.
Aliquots of the samples used in Fig. 1a–c were analyzed by immunoblotting using anti-Flag and anti-HA antibody (A,C,E) and RT-qPCR (B,D,F) (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. G, Detection of HIV-2/SIVmac HA- Vpx and endogenous SAMHD1 in HIV-2/SIVmac Vpx- transduced EA.hy926 cells. (One representative immunoblotting result out of n = 2 independent biological experiments is shown.) H, Transduced EA.hy926 cells were transfected with ISD (2 μg/mL) for 12h. Total RNA was prepared and analyzed for the transcriptional level of the indicated genes by RT-qPCR (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test.
Extended Data Fig. 4 Vpx inhibits NF-κB signaling induced by cGAS-STING from a lentiviral expression vector or virus infection.
A,B, Vpx expressed from a lentiviral expression vector inhibited NF-κB signaling induced by cGAS-STING. HEK293T cells were co-transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and pLVX-HIV-2 ROD Vpx as indicated. After 24h, NF-κB promoter activity was analyzed (A), and the cell lysates were analyzed by immunoblotting with anti-Flag or anti-HA antibody (B) (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. C,D, Vpx expressed from an SIVmac infectious clone inhibited NF-κB signaling induced by cGAS-STING. HEK293T cells were transfected with SIVmac wild-type or SIVmacΔVpx for 12h. Subsequently the NF-κB promoter, pRL-TK Renilla, Myc-cGAS, and STING-Flag vectors were transfected as indicated for 24h. NF-κB promoter activity was analyzed by luciferase reporter assays (C), and the cell lysates were analyzed by immunoblotting with antibody targeting Pr55Gag or Flag (D) (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. E,F, Vpx expressed during viral infection inhibited NF-κB signaling induced by cGAS-STING. EA.hy926 cells were infected with an equal amount of SIVmac or SIVmacΔVpx virus for 12h. Subsequently, the cells were transfected with ISD (2 μg/mL) for another 12h. Cell lysates were analyzed by immunoblotting with anti-SAMHD1 and anti-CAp27 antibodies (E). (A representative immunoblotting result out of n = 3 independent biological experiments is shown.) Total RNA was prepared and analyzed for the transcriptional level of the indicated genes by RT-qPCR (F) (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. G, Schematic description of the experimental procedure. H, HEK293T cells were infected with an equal amount of SIVmac or SIVmacΔVpx virus for 12h. Subsequently, the cells were transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, and STING-Flag as indicated. NF-κB promoter activity was measured 24h after transfection, and the cell lysates were analyzed by immunoblotting with the indicated antibodies (n = 3 independent biological experiments). Means and standard deviations are shown. The statistical significance analyses were performed using a two-sided unpaired t-test. I, BMDCs were infected with SIVmac or SIVmacΔVpx. After 4 h, the medium was changed, and the cells were cultured with or without STING agonist (20 μM CDA) for another 36h. Then BMDCs were harvested, and CD86 protein expression was tested by flow cytometry (n = 3 independent biological experiments). The gating strategy is shown. Numbers indicate the percentage in the gate. The bar graph shows the increased numbers of CD86 positive cells after STING agonist treatment (number of CD86 positive cells treated with STING agonist subtracts number of CD86 positive cells treated without STING agonist). Bar graph data are from n = 3 independent biological experiments. Means and standard deviations are shown. The statistical significance analyses were performed using a two- sided unpaired t-test.
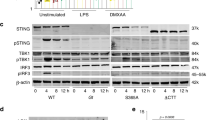
Extended Data Fig. 5 Vpx suppressed cGAS- STING function is SAMHD1 independent and Inhibition of cGAS- STING-mediated NF-κB signaling is a conserved function of Vpx proteins from diverse HIV-2 and SIV viruses.
A, Aliquots of the samples used were analyzed by RT-qPCR. RNA was isolated, followed by detection of NFKBIA, IER3, and CXCL8 mRNA levels by RT-qPCR (n = 3 independent biological experiments). Means and standard deviations are shown. The statistical significance analyses were performed using a two- sided unpaired t-test. B, The graph represents the location of siRNA-1, siRNA-2, and shRNA targeting in the SAMHD1 coding region. C, HEK293T cells were transfected with siNT, siSAMHD1-1, or siSAMHD1-2 for 24 h and then transfected NF- κB-Luc or pRL-TK Renilla, together with STING-Flag and Myc- cGAS, empty vector or SIVmac Vpx. Promoter transactivation was analyzed by luciferase reporter assay, and the protein expression levels were analyzed by immunoblotting with antibody targeting SAMHD1 or GAPDH (n = 3 independent biological experiments). One representative immunoblotting result out of n = 3 independent experiments is shown. Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. D, HEK293T cells were co-transfected with NF- κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and HIV/SIV HA-Vpx as indicated. The cell lysates were detected by immunoblotting with anti-SAMHD1, anti- Flag, or anti-HA antibody. (One representative immunoblotting result out of n = 3 independent biological experiments is shown.) E, The mRNA levels of NFKBIA, CXCL8, and CXCL10 were analyzed by RT-qPCR (n = 3 independent biological experiments). Means and standard deviations are shown. The statistical significance analyses were performed using a two-sided unpaired t-test.
Extended Data Fig. 6 The DCAF1 binding- defective Vpx mutant Q76R also impairs cGAS- STING-triggered NF-κB activation and silencing DCAF1 does not affect Vpx-mediated cGAS- STING inhibition.
A,B, HEK293T cells were co-transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING- Flag, and increasing amounts of SIVmac HA-Vpx (wild-type or Q76R) as indicated. At 24 h after transfection, the cell lysates were detected by immunoblotting with anti-SAMHD1, anti- Flag, or anti-HA antibody (A). The NF-κB promoter activity was analyzed by luciferase reporter assays (B). C,D, HEK293T cells were co-transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and SIVmac HA- Vpx (wild-type or Q76R) as indicated. The cell lysates were detected by immunoblotting with anti-SAMHD1, anti-Flag, or anti-HA antibody 24h after transfection (C). The mRNA levels of NFKBIA, IER3, GADD45B, and CXCL8 were analyzed by RT- qPCR (D). E,F, HEK293T cells were co-transfected with the NF- κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and increasing amounts of HIV-2 ROD HA-Vpx (wild-type or Q76R) as indicated. After 24h, the cell lysates were detected by immunoblotting with anti-SAMHD1, anti- Flag, or anti-HA antibody (E). The NF-κB promoter activity was analyzed by luciferase reporter assays (F). G,H, HEK293T cells were co-transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and HIV-2 ROD HA-Vpx (wild-type or Q76R) as indicated. The cell lysates were detected by immunoblotting with anti-SAMHD1, anti-Flag, or anti-HA antibody 24 h after transfection (G). The mRNA levels of NFKBIA, IER3, GADD45B, CXCL10, and CXCL8 were analyzed by RT-qPCR (H). I, HEK293T cells transduced with shNT or shDCAF1 were generated. Transduced HEK293T cells were co- transfected with the NF-κB promoter luciferase vector, pRL-TK Renilla, Myc-cGAS, STING-Flag, and HA-Vpx (SIVmac, HIV-2 ROD, or HIV-2 7312A) as indicated. The cell lysates were detected by immunoblotting with anti-DCAF1, anti-Flag, or anti-HA antibody 24h after transfection. J, NF-κB promoter activity was analyzed by luciferase reporter assay using parallel samples prepared as described above. (One representative immunoblotting result out of n = 3 independent biological experiments is shown (A, C, E, G, I)). Data are from n = 3 independent biological experiments. Means and standard deviations (B, D, F, H, J) are shown. The statistical significance analyses were performed using a two-sided unpaired t-test.
Extended Data Fig. 7 SIVmac Vpx interacts with endogenous STING.
A, The STING CTD (C-terminal domain) from different primates is conserved. Alignment of the STING CTD sequences of STINGs from Homo sapiens (NP_938023), Mus musculus (NP_082537), Macaca mulatta (XP_014996496), Macaca fascicularis (XP_005557992), Macaca nemestrina (XP_011714679), Cercocebus atys (XP_011945838), Chlorocebus sabaeus (XP_008012825), Pan troglodytes (XP_016809410), Pan paniscus (XP_003829248), Pongo abelii (XP_002815998), Papio anubis (XP_021795910), Rhinopithecus roxellana (XP_010386421), Mandrillus leucophaeus (XP_011852614), and Colobus angolensis palliatus (XP_011790719). Sequence alignment was carried out using Mega 5.01 software. B, HEK293T cells were co- transfected with SIVmacΔVpx or VSV-G together with empty vector, Vpx WT, or Vpx RS51/52AA. At 12h after transfection, the culture medium was changed. At 36h after transfection, the supernatant was filtered and used to infect Jurkat cells. At 12h after infection, cell lysates were prepared and immunoprecipitated using anti-HA beads. Precipitated samples were separated by SDS-PAGE, transferred to nitrocellulose membranes, and reacted with an anti-HA antibody to detect HA- Vpx or an anti-STING antibody to detect STING. GAPDH was used as a loading control. (One representative immunoblotting result out of n = 2 independent experiments is shown.) C, CD4+ T cells were infected with equal amounts of SIVmacΔVpx, SIVmacΔVpx+Vpx WT, SIVmacΔVpx+Vpx ET16/17AA, or SIVmacΔVpx+Vpx RS51/52AA virus for 4h; then the viruses were removed by changing the cells into fresh medium containing the 30 μM STING agonist or DMSO and incubating for another 8h. Total RNA was prepared from the harvested cells and analyzed for the transcriptional level of the indicated genes by RT-qPCR. Different shapes represent different donor, with three independent experiments for each donor. A single mean value, depicted by a single horizontal line, is shown and marked in red. Means and standard deviations are presented.
Extended Data Fig. 8 Summary of how Vpx helix mutations affect cGAS-STING inhibition and identification of STING mutants defective for NF-κB signaling.
A, Schematic representation of the SIVmac Vpx mutants used in this study. Relative cGAS-STING inhibition (right panel) is shown in the red chart. The inhibitory activity of Vpx was set as 100% (n = 3 independent biological experiments). B, Structural representation of the Vpx/SAMHD1-CTD/DCAF1- CTD ternary complex. Color codes for the proteins are indicated. Functionally important residues in Vpx for cGAS- STING inhibition are shown in red. The zinc ion is shown as an orange sphere, and HHCC residues of Vpx are in blue. DCAF1 is shown in yellow; cylinders represent helices in SAMHD1 (purple) and Vpx (green). The structure was prepared using PyMOL (http://www.pymol.org/). C, Alignment of amino acids in helices 1, 2, and 3 of HIV-2/SIV Vpx proteins. Zinc-binding residues are shown in blue. Residues in Vpx that are required for cGAS-STING inhibition are shown in red. Sequence alignment was carried out using Mega 6 software. D, Identification of STING residues important for NF-κB signaling. HEK293T cells were transfected with NF-κB-Luc and the cGAS- STING expression vector with STING or a STING mutant. pRL- TK Renilla was used as the internal control. Transactivation of the luciferase reporter was determined 24 h after transfection (n = 3 independent biological experiments). Means and standard deviations are presented. A two-sided unpaired t- test was performed. E, A proposed model for STING-mediated NF-κB pathway activation that is antagonized by Vpx. Vpx binds to critical residues (329–334 amino acids) in STING that are important for STING-mediated NF-κB signaling.
Extended Data Fig. 9 Detection of intracellular p50 by immunofluorescent staining.
A, HEK293T cells were transfected with Myc-cGAS and STING- Flag, in the presence or absence of HA-Vpx. Empty vector was used as a control. Immunofluorescent staining was carried out using anti-p50 antibody to detect p50. DAPI staining was performed to show the nuclei (representative images out of n = 4 independent biological experiments). The arrowheads show the p50 nuclear localization under cGAS-STING stimulation. B, Relative p50 nuclear/cytoplasmic localization was calculated and is shown as a bar graph. The results in the presence of cGAS-STING and in the absence of Vpx were set to 100%. Means and standard deviations are presented. A two- sided unpaired t-test was performed.
Extended Data Fig. 10 Influence of SIVmac Vpx on STING-mediated NF- κB signaling and viral infection.
A,B, Jurkat cells were infected with CAp27-normalized SIVmac or SIVmacΔVpx virus for 48h. The productive viral infection was analyzed by flow cytometry (n = 3 independent biological experiments) using antibody against CAp27 (A). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. Intracellular Pr55Gag was analyzed by immunoblotting with the anti-CA antibody. GAPDH was used as a loading control (B) (Immunoblotting is a representative of n = 3 independent biological experiments.) C, Influence of SIVmac Vpx on STING- mediated p50 nuclear translocation. Jurkat cells were infected with equal amounts of SIVmacΔVpx or SIVmacΔVpx+HA-Vpx, SIVmacΔVpx+Vpx ET16/17AA, or SIVmacΔVpx+Vpx RS51/52AA virus and treated with STING agonist as in Fig. 5. Cells were harvested, and the nuclear and cytoplasmic fractions were separated 12h after infection. Proteins were analyzed by immunoblotting using anti-p50, anti-HA, anti-IRF3, anti- GAPDH, or anti-histone antibody. Immunoblotting is representative of n = 2 independent biological experiments. D,E, STING is critical for Vpx-mediated suppression of the STING agonist-triggered anti-viral effect. STING-silenced Jurkat (shSTING) or control (shNT) cells were monitored by immunoblotting using anti-STING and anti-GAPDH antibody (D) (A representative immunoblotting result out of n = 2 independent biological experiments is shown.) STING-silenced Jurkat (shSTING) or control (shNT) cells were infected with equal amounts of SIVmac or SIVmacΔVpx virus. The medium was changed 4h later and replaced with fresh medium containing DMSO or STING agonist. Supernatants from the Jurkat cells were collected at 48h and 72h after infection and analyzed for viral infectivity using the TZM-bl luciferase reporter assay (E) (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. F, Jurkat cells were infected with equal amounts of SIVmac or SIVmacΔVpx virus; 4h later, the medium was changed, and SeV (20 HA/mL) was added to the medium. Cells and total mRNA were prepared, and mRNA was analyzed by RT-qPCR to determine the transcription levels of the indicated genes (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. G, Jurkat cells were infected with SIVmacΔVpx or SIVmacΔVpx+HA-Vpx virus for 12h, and then infected with HIV-1 NL4-3ΔEnvGFP virus for 48h. Infected cells were harvested, and GFP-positive cells were tested by flow cytometry (n = 3 independent biological experiments). Means and standard deviations are presented. The statistical significance analyses were performed using a two-sided unpaired t-test. H, CD4+ T cells were infected with equal amounts of SIVmacΔVpx incorporated with Vpx WT, ET16/17AA, or RS51/52AA mutant virus and treated with STING agonist or not. Total cells were harvested, and the protein expression levels were analyzed by immunoblotting with anti-IRF3-p, anti-IFIT3, anti-ISG15, anti-STING, anti-cGAS, anti-HA, or anti-GAPDH antibody. A representative immunoblotting result out of n = 2 independent biological experiments is shown.
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Source data
Source Data Fig. 1
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Su, J., Rui, Y., Lou, M. et al. HIV-2/SIV Vpx targets a novel functional domain of STING to selectively inhibit cGAS–STING-mediated NF-κB signalling. Nat Microbiol 4, 2552–2564 (2019). https://doi.org/10.1038/s41564-019-0585-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0585-4
This article is cited by
-
TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion
Signal Transduction and Targeted Therapy (2023)
-
HIV-2/SIV Vpx antagonises NF-κB activation by targeting p65
Retrovirology (2022)
-
Multifaceted functions of STING in human health and disease: from molecular mechanism to targeted strategy
Signal Transduction and Targeted Therapy (2022)
-
Vpx enhances innate immune responses independently of SAMHD1 during HIV-1 infection
Retrovirology (2021)
-
Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins
Signal Transduction and Targeted Therapy (2021)