Abstract
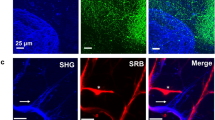
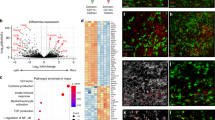
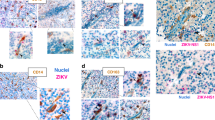
The protozoan parasite Toxoplasma gondii is thought to exploit monocyte trafficking to facilitate dissemination across endothelial barriers such as the blood–brain barrier. Here, we analysed the migration of parasitized monocytes in model endothelial and interstitial environments. We report that infection enhanced monocyte locomotion on the surface of endothelial cells, but profoundly inhibited monocyte transmigration across endothelial barriers. By contrast, infection robustly increased monocyte and macrophage migration through collagen-rich tissues in a Rho–ROCK-dependent manner consistent with integrin-independent interstitial migration. We further demonstrated that the secreted T. gondii protein kinase ROP17 was required for enhanced tissue migration. In vivo, ROP17-deficient parasites failed to upregulate monocyte tissue migration and exhibited an early dissemination delay, leading to prolonged mouse survival. Our findings indicate that the parasite-induced changes in monocyte motility primarily facilitate the transport of T. gondii through tissues and promote systemic dissemination, rather than shuttle parasites across the blood–brain barrier via extravasation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and analysed during this study are included within the paper and the associated Supplementary Information.
References
Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet 363, 1965–1976 (2004).
Santiago-Tirado, F. H. & Doering, T. L. False friends: phagocytes as Trojan horses in microbial brain infections. PLoS Pathog. 13, e1006680 (2017).
Harker, K. S., Ueno, N. & Lodoen, M. B. Toxoplasma gondii dissemination: a parasite’s journey through the infected host. Parasite Immunol. 37, 141–149 (2015).
Dubey, J. P. Bradyzoite-induced murine toxoplasmosis: stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J. Eukaryot. Microbiol. 44, 592–602 (1997).
Dubey, J. P., Speer, C. A., Shen, S. K., Kwok, O. C. & Blixt, J. A. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J. Parasitol. 83, 870–882 (1997).
Gregg, B. et al. Replication and distribution of Toxoplasma gondii in the small intestine after oral infection with tissue cysts. Infect. Immun. 81, 1635–1643 (2013).
Joeris, T., Muller-Luda, K., Agace, W. W. & Mowat, A. M. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 10, 845–864 (2017).
Dunay, I. R. et al. Gr1+ inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29, 306–317 (2008).
Konradt, C. et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat. Microbiol. 1, 16001 (2016).
Ley, K., Laudanna, C., Cybulsky, M. I. & Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007).
Courret, N. et al. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood 107, 309–316 (2006).
Ueno, N. et al. Real-time imaging of Toxoplasma-infected human monocytes under fluidic shear stress reveals rapid translocation of intracellular parasites across endothelial barriers. Cell. Microbiol. 16, 580–595 (2014).
Lambert, H., Dellacasa-Lindberg, I. & Barragan, A. Migratory responses of leukocytes infected with Toxoplasma gondii. Microbes Infect. 13, 96–102 (2011).
Ueno, N. & Lodoen, M. B. From the blood to the brain: avenues of eukaryotic pathogen dissemination to the central nervous system. Curr. Opin. Microbiol. 26, 53–59 (2015).
Cook, J. H., Ueno, N. & Lodoen, M. B. Toxoplasma gondii disrupts β1 integrin signaling and focal adhesion formation during monocyte hypermotility. J. Biol. Chem. 293, 3374–3385 (2018).
Harker, K. S. et al. Toxoplasma gondii modulates the dynamics of human monocyte adhesion to vascular endothelium under fluidic shear stress. J. Leukoc. Biol. 93, 789–800 (2013).
Olafsson, E. B., Varas-Godoy, M. & Barragan, A. Toxoplasma gondii infection shifts dendritic cells into an amoeboid rapid migration mode encompassing podosome dissolution, secretion of TIMP-1, and reduced proteolysis of extracellular matrix. Cell. Microbiol. 20, e12808 (2018).
Lambert, H., Vutova, P. P., Adams, W. C., Loré, K. & Barragan, A. The Toxoplasma gondii-shuttling function of dendritic cells is linked to the parasite genotype. Infect. Immun. 77, 1679–1688 (2009).
Fuks, J. M. et al. GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PLoS Pathog. 8, e1003051 (2012).
Weidner, J. M. et al. Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell. Microbiol. 15, 1735–1752 (2013).
Daniels, B. P. et al. Immortalized human cerebral microvascular endothelial cells maintain the properties of primary cells in an in vitro model of immune migration across the blood brain barrier. J. Neurosci. Methods 212, 173–179 (2013).
van Kooyk, Y. & Figdor, C. G. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr. Opin. Cell Biol. 12, 542–547 (2000).
Onken, M. D. et al. Endothelial monolayers and transendothelial migration depend on mechanical properties of the substrate. Cytoskeleton (Hoboken) 71, 695–706 (2014).
Belin, B. J., Goins, L. M. & Mullins, R. D. Comparative analysis of tools for live cell imaging of actin network architecture. Bioarchitecture 4, 189–202 (2014).
Charras, G. & Paluch, E. Blebs lead the way: how to migrate without lamellipodia. Nat. Rev. Mol. Cell Biol. 9, 730–736 (2008).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Kanatani, S., Uhlen, P. & Barragan, A. Infection by Toxoplasma gondii induces amoeboid-like migration of dendritic cells in a three-dimensional collagen matrix. PLoS ONE 10, e0139104 (2015).
Wolf, K. et al. Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 20, 931–941 (2009).
Weber, M. & Sixt, M. Live cell imaging of chemotactic dendritic cell migration in explanted mouse ear preparations. Methods Mol. Biol. 1013, 215–226 (2013).
Cougoule, C. et al. Blood leukocytes and macrophages of various phenotypes have distinct abilities to form podosomes and to migrate in 3D environments. Eur. J. Cell Biol. 91, 938–949 (2012).
Van Goethem, E., Poincloux, R., Gauffre, F., Maridonneau-Parini, I. & Le Cabec, V. Matrix architecture dictates three-dimensional migration modes of human macrophages: differential involvement of proteases and podosome-like structures. J. Immunol. 184, 1049–1061 (2010).
Lämmermann, T. & Germain, R. N. The multiple faces of leukocyte interstitial migration. Semin. Immunopathol. 36, 227–251 (2014).
Shang, X. et al. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 19, 699–710 (2012).
Martiny-Baron, G. et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 268, 9194–9197 (1993).
Rizvi, S. A. et al. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem. Biol. 16, 1158–1168 (2009).
Nolen, B. J. et al. Characterization of two classes of small molecule inhibitors of Arp2/3 complex. Nature 460, 1031–1034 (2009).
Hunter, C. A. & Sibley, L. D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 10, 766–778 (2012).
Hakimi, M. A., Olias, P. & Sibley, L. D. Toxoplasma effectors targeting host signaling and transcription. Clin. Microbiol. Rev. 30, 615–645 (2017).
Etheridge, R. D. et al. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15, 537–550 (2014).
Hodge, R. G. & Ridley, A. J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 17, 496–510 (2016).
Talevich, E. & Kannan, N. Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evolut. Biol. 13, 117 (2013).
Guilluy, C., Garcia-Mata, R. & Burridge, K. Rho protein crosstalk: another social network? Trends Cell Biol. 21, 718–726 (2011).
Fox, B. A. et al. The Toxoplasma gondii Rhoptry kinome is essential for chronic infection. mBio 7, e00193-16 (2016).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Renkawitz, J. & Sixt, M. Mechanisms of force generation and force transmission during interstitial leukocyte migration. EMBO Rep. 11, 744–750 (2010).
Hakansson, S., Charron, A. J. & Sibley, L. D. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20, 3132–3144 (2001).
Panas, M. W. et al. Translocation of dense granule effectors across the parasitophorous vacuole membrane in Toxoplasma-infected cells requires the activity of ROP17, a rhoptry protien kinase. Preprint at https://doi.org/10.1101/613208 (2019).
Weksler, B. B. et al. Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 19, 1872–1874 (2005).
Kreisel, D. et al. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl Acad. Sci. USA 107, 18073–18078 (2010).
Zinselmeyer, B. H. et al. Chapter 16. Two-photon microscopy and multidimensional analysis of cell dynamics. Methods Enzymol. 461, 349–378 (2009).
Kowarz, E., Loscher, D. & Marschalek, R. Optimized Sleeping Beauty transposons rapidly generate stable transgenic cell lines. Biotechnol. J. 10, 647–653 (2015).
Alaganan, A., Fentress, S. J., Tang, K., Wang, Q. & Sibley, L. D. Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc. Natl Acad. Sci. USA 111, 1126–1131 (2014).
Schaefer, L. H., Schuster, D. & Herz, H. Generalized approach for accelerated maximum likelihood based image restoration applied to three-dimensional fluorescence microscopy. J. Microsc. 204, 99–107 (2001).
Acknowledgements
We thank J. A. Cooper for constructive comments; R. Klein and T. Doering for sharing the hCMEC/D3 cells; T. Fehniger for facilitating the acquisition of primary human monocytes; F. Santiago and H. Salimi for advice regarding the BBB model; S. Kim, L. Yang and the Washington University School of Medicine In vivo Imaging Core for technical support and fluorescent reporter mice for the intravital imaging experiments; and G. Randolph for generously sharing the CX3CR1GFP/+ mice. Work was supported in part by grants from the National Science Foundation to L.L.D. (DGE-1143954), and from the National Institutes of Health to M.J.M. (R01AI077600) and L.D.S. (AI034036).
Author information
Authors and Affiliations
Contributions
L.L.D. and L.D.S. designed the experiments and wrote the manuscript. N.G.J. generated the T. gondii strains used for time-lapse microscopy. Q.W. collaborated on the design and execution of animal studies. M.D.O. collaborated on the design and interpretation of the Rho GTPase inhibitor studies. L.L.D. and M.J.M. designed and performed the two-photon imaging experiments. L.L.D. performed all other experiments and analysed the data. L.D.S. supervised the study. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Supplementary Video legends, Supplementary Tables 1–3 and Supplementary References.
Supplementary Video 1
Uninfected THP-1 monocyte motility on hCMEC/D3 (shown in Fig. 2).
Supplementary Video 2
Infected THP-1 monocyte motility on hCMEC/D3 (shown in Fig. 2).
Supplementary Video 3
Infected THP-1 monocyte motility on hCMEC/D3 (shown in Fig. 2).
Supplementary Video 4
Uninfected CX3CR1GFP/+ cell migrating in spleen (shown in Fig. 6).
Supplementary Video 5
Infected CX3CR1GFP/+ cell migrating in spleen (shown in Fig. 6).
Supplementary Video 6
Infected CX3CR1GFP/+ cell migrating in spleen (shown in Fig. 6).
Rights and permissions
About this article
Cite this article
Drewry, L.L., Jones, N.G., Wang, Q. et al. The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat Microbiol 4, 1951–1963 (2019). https://doi.org/10.1038/s41564-019-0504-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0504-8
This article is cited by
-
Salmonella manipulates macrophage migration via SteC-mediated myosin light chain activation to penetrate the gut-vascular barrier
The EMBO Journal (2024)
-
Toxoplasma protein export and effector function
Nature Microbiology (2024)
-
Toxoplasma rhoptry proteins that affect encephalitis outcome
Cell Death Discovery (2023)
-
Immune response and pathogen invasion at the choroid plexus in the onset of cerebral toxoplasmosis
Journal of Neuroinflammation (2022)
-
Toxoplasma gondii infection and its implications within the central nervous system
Nature Reviews Microbiology (2021)