Abstract
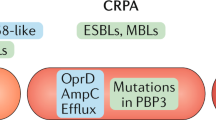
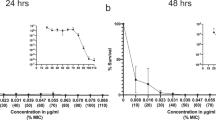
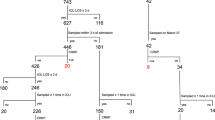
Antibiotic-resistant bacteria are a significant threat to human health, with one estimate suggesting they will cause 10 million worldwide deaths per year by 2050, surpassing deaths due to cancer1. Because new antibiotic development can take a decade or longer, it is imperative to effectively use currently available drugs. Antibiotic combination therapy offers promise for treating highly resistant bacterial infections, but the factors governing the sporadic efficacy of such regimens have remained unclear. Dogma suggests that antibiotics ineffective as monotherapy can be effective in combination2. Here, using carbapenem-resistant Enterobacteriaceae (CRE) clinical isolates, we reveal the underlying basis for the majority of effective combinations to be heteroresistance. Heteroresistance is a poorly understood mechanism of resistance reported for different classes of antibiotics3,4,5,6 in which only a subset of cells are phenotypically resistant7. Within an isolate, the subpopulations resistant to different antibiotics were distinct, and over 88% of CRE isolates exhibited heteroresistance to multiple antibiotics (‘multiple heteroresistance’). Combinations targeting multiple heteroresistance were efficacious, whereas those targeting homogenous resistance were ineffective. Two pan-resistant Klebsiella isolates were eradicated by combinations targeting multiple heteroresistance, highlighting a rational strategy to identify effective combinations that employs existing antibiotics and could be clinically implemented immediately.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in this Article are presented in the paper or the Supplementary Information. Any additional data can be requested from the corresponding author.
References
Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations (Review on Antimicrobial Resistance, 2014); https://amr-review.org/Publications.html
Micek, S. T. et al. Empiric combination antibiotic therapy is associated with improved outcome against sepsis due to Gram-negative bacteria: a retrospective analysis. Antimicrob. Agents Chemother. 54, 1742–1748 (2010).
Alexander, H. E. & Leidy, G. Mode of action of streptomycin on type B Hemophilis influenzae: nature of resistant variants. J. Exp. Med 85, 607–621 (1947).
Ott, J. L., Turner, J. R. & Mahoney, D. F. Lack of correlation between beta-lactamase production and susceptibility to cefamandole or cefoxitin among spontaneous mutants of Enterobacteriaceae. Antimicrob. Agents Chemother. 15, 14–19 (1979).
Søgaard, P. & Gahrn-Hansen, B. Population analysis of susceptibility to ciprofloxacin and nalidixic acid in Staphylococcus, Pseudomonas aeruginosa, and Enterobacteriaceae. Acta Pathol. Microbiol. Immunol. Scand. B 94, 351–356 (1986).
Band, V. I. et al. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat. Microbiol. 1, 16053 (2016).
El-Halfawy, O. M. & Valvano, M. A. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin. Microbiol. Rev. 28, 191–207 (2015).
Antibiotic Resistance Threats in the United States, 2013 (US CDC, 2013).
Guh, A. Y. et al. Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 314, 1479–1487 (2015).
Chen, L., Todd, R., Kiehlbauch, J., Walters, M. & Kallen, A. Pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae — Washoe County, Nevada, 2016. Morb. Mortal. Wkly Rep. 66, 33 (2016).
Multi-site Gram-negative Surveillance Initiative (US CDC, 2016).
Brauner, A., Fridman, O., Gefen, O. & Balaban, N. Q. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nat. Rev. Microbiol. 14, 320–330 (2016).
Keren, I., Minami, S., Rubin, E. & Lewis, K. Characterization and transcriptome analysis of Mycobacterium tuberculosis persisters. mBio 2, e00100–e00111 (2011).
Wakamoto, Y. et al. Dynamic persistence of antibiotic-stressed mycobacteria. Science 339, 91–95 (2013).
Napier, B. A., Band, V., Burd, E. M. & Weiss, D. S. Colistin heteroresistance in Enterobacter cloacae is associated with cross-resistance to the host antimicrobial lysozyme. Antimicrob. Agents Chemother. 58, 5594–5597 (2014).
Kang K. N. et al. Colistin heteroresistance in Enterobacter cloacae is regulated by PhoPQ-dependent 4-amino-4-deoxy-l-arabinose addition to lipid A. Mol. Microbiol. https://doi.org/10.1111/mmi.14240 (2019).
Yang, T. Y., Lu, P. L. & Tseng, S. P. Update on fosfomycin-modified genes in Enterobacteriaceae. J. Microbiol. Immunol. Infect. 52, 9–21 (2017).
Normark, S. Beta-lactamase induction in Gram-negative bacteria is intimately linked to peptidoglycan recycling. Microb. Drug Resist. 1, 111–114 (1995).
Nicoloff, H., Hjort, K., Levin, B. R. & Andersson, D. I. The high prevalence of antibiotic heteroresistance in pathogenic bacteria is mainly caused by gene amplification. Nat. Microbiol. 4, 504–514 (2019).
Ballestero-Téllez, M. et al. Role of inoculum and mutant frequency on fosfomycin MIC discrepancies by agar dilution and broth microdilution methods in Enterobacteriaceae. Clin. Microbiol. Infect. 23, 325–331 (2017).
Paul, M., Benuri-Silbiger, I., Soares-Weiser, K. & Leibovici, L. Beta lactam monotherapy versus beta lactam–aminoglycoside combination therapy for sepsis in immunocompetent patients: systematic review and meta-analysis of randomised trials. BMJ 328, 668 (2004).
Leibovici, L. et al. Monotherapy versus beta-lactam–aminoglycoside combination treatment for Gram-negative bacteremia: a prospective, observational study. Antimicrob. Agents Chemother. 41, 1127–1133 (1997).
Siegman-Igra, Y., Ravona, R., Primerman, H. & Giladi, M. Pseudomonas aeruginosa bacteremia: an analysis of 123 episodes, with particular emphasis on the effect of antibiotic therapy. Int. J. Infect. Dis. 2, 211–215 (1998).
Crabtree, T. D., Pelletier, S. J., Gleason, T. G., Pruett, T. L. & Sawyer, R. G. Analysis of aminoglycosides in the treatment of Gram-negative infections in surgical patients. Arch. Surg. 134, 1293–1299 (1999).
Montravers, P. et al. Diagnostic and therapeutic management of nosocomial pneumonia in surgical patients: results of the Eole study. Crit. Care Med. 30, 368–375 (2002).
Grasela, T. H. et al. A nationwide survey of antibiotic prescribing patterns and clinical outcomes in patients with bacterial pneumonia. DICP 24, 1220–1225 (1990).
Odds, F. C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52, 1 (2003).
Doern, C. D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J. Clin. Microbiol. 52, 4124–4128 (2014).
Tamma, P. D., Cosgrove, S. E. & Maragakis, L. L. Combination therapy for treatment of infections with Gram-negative bacteria. Clin. Microbiol. Rev. 25, 450–470 (2012).
Paul, M. et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J. Antimicrob. Chemother. 69, 2305–2309 (2014).
Etest application guide (bioMérieux, 2012); https://go.nature.com/2WavcTf
Datsenko, K. A. & Wanner, B. L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl Acad. Sci. USA 97, 6640–6645 (2000).
Acknowledgements
The authors thank A. Grakoui, H. Ratner and W. Shafer for critical reading of the manuscript, and C. Bower and the GA EIP/MuGSI staff for providing CRE isolates. D.A.H. is supported by a postdoctoral research fellowship from the Cystic Fibrosis Foundation. E.X.S. is supported by T32 training grant AI106699 from the National Institutes of Health (NIH). D.S.W. is supported by a Burroughs Wellcome Fund Investigator in the Pathogenesis of Infectious Disease award and NIH grant AI141883. The GA EIP is funded by the Centers for Disease Control and Prevention. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the CDC.
Author information
Authors and Affiliations
Contributions
V.I.B. and D.A.H. performed the majority of the experiments. S.J., E.X.S. and J.E.W. contributed to the studies quantifying the prevalence of heteroresistance. E.M.B. performed clinical susceptibility testing. S.W.S., M.M.F. and J.J. contributed to CRE isolate collection, identification and characterization. V.I.B., D.A.H. and D.S.W. conceived of the experiments and wrote the manuscript, which all authors reviewed and edited.
Corresponding author
Ethics declarations
Competing interests
V.I.B., D.A.H. and D.S.W. are listed authors on a provisional patent that has been filed related to the work described here.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–12, Supplementary Tables 1–2, Supplementary File 1
Rights and permissions
About this article
Cite this article
Band, V.I., Hufnagel, D.A., Jaggavarapu, S. et al. Antibiotic combinations that exploit heteroresistance to multiple drugs effectively control infection. Nat Microbiol 4, 1627–1635 (2019). https://doi.org/10.1038/s41564-019-0480-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0480-z
This article is cited by
-
Bacteria can compensate the fitness costs of amplified resistance genes via a bypass mechanism
Nature Communications (2024)
-
Multiple heteroresistance to tigecycline and colistin in Acinetobacter baumannii isolates and its implications for combined antibiotic treatment
Journal of Biomedical Science (2023)
-
Ceftazidime/avibactam combined with colistin: a novel attempt to treat carbapenem-resistant Gram-negative bacilli infection
BMC Infectious Diseases (2023)
-
Synthesis of a covalent organic framework with hetero-environmental pores and its medicine co-delivery application
Nature Communications (2023)
-
The physiology and genetics of bacterial responses to antibiotic combinations
Nature Reviews Microbiology (2022)