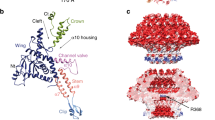
Abstract
Phage tail fibres are elongated protein assemblies capable of specific recognition of bacterial surfaces during the first step of viral infection1,2,3,4. The folding of these complex trimeric structures often requires a phage-encoded tail fibre assembly (Tfa) protein5,6,7. Despite the wide occurrence of Tfa proteins, their functional mechanism has not been elucidated. Here, we investigate the tail fibre and Tfa of Escherichia coli phage Mu. We demonstrate that Tfa forms a stable complex with the tail fibre, and present a 2.1 Å resolution X-ray crystal structure of this complex. We find that Tfa proteins are comprised of two domains: a non-conserved N-terminal domain that binds to the C-terminal region of the fibre and a conserved C-terminal domain that probably mediates fibre oligomerization and assembly. Tfa forms rapidly exchanging multimers on its own, but not a stable trimer, implying that Tfa does not specify the trimeric state of the fibre. We propose that the key conserved role of Tfa is to ensure that fibre assembly and multimerization initiates at the C terminus, ensuring that the intertwined and repetitive structural elements of fibres come together in the correct sequence. The universal importance of correctly aligning the C termini of phage fibres is highlighted by our work.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and the Supplementary Information, or from the corresponding authors upon request. The structure of the TfibMu:TfaMu complex obtained in the current study is available in the PDB with the following accession code: PDB ID 5YVQ.
References
van de Putte, P., Cramer, S. & Giphart-Gassler, M. Invertible DNA determines host specificity of bacteriophage Mu. Nature 286, 218–222 (1980).
Wilson, J. H., Luftig, R. B. & Wood, W. B. Interaction of bacteriophage T4 tail fiber components with a lipopolysaccharide fraction from Escherichia coli. J. Mol. Biol. 51, 423–434 (1970).
Le, S. et al. Mapping the tail fiber as the receptor binding protein responsible for differential host specificity of Pseudomonas aeruginosa bacteriophages PaP1 and JG004. PLoS ONE 8, e68562 (2013).
Grundy, F. J. & Howe, M. M. Involvement of the invertible G segment in bacteriophage Mu tail fiber biosynthesis. Virology 134, 296–317 (1984).
Bartual, S. G., Garcia-Doval, C., Alonso, J., Schoehn, G. & van Raaij, M. J. Two-chaperone assisted soluble expression and purification of the bacteriophage T4 long tail fibre protein gp37. Protein Expr. Purif. 70, 116–121 (2010).
Hashemolhosseini, S., Stierhof, Y. D., Hindennach, I. & Henning, U. Characterization of the helper proteins for the assembly of tail fibers of coliphages T4 and lambda. J. Bacteriol. 178, 6258–6265 (1996).
Leiman, P. G. et al. Morphogenesis of the T4 tail and tail fibers. Virol. J. 7, 355 (2010).
Sandulache, R., Prehm, P. & Kamp, D. Cell wall receptor for bacteriophage Mu G(+). J. Bacteriol. 160, 299–303 (1984).
Bartual, S. G. et al. Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proc. Natl Acad. Sci. USA 107, 20287–20292 (2010).
Granell, M., Namura, M., Alvira, S., Kanamaru, S. & van Raaij, M. J. Crystal structure of the carboxy-terminal region of the bacteriophage T4 proximal long tail fiber protein Gp34. Viruses 9, E168 (2017).
Abedon, S. T., Garcia, P., Mullany, P. & Aminov, R. Editorial: Phage therapy: past, present and future. Front Microbiol 8, 981 (2017).
Denyes, J. M. et al. Modified Bacteriophage S16 Long Tail Fiber Proteins for Rapid and Specific Immobilization and Detection of Salmonella Cells. Appl. Environ. Microbiol. 83, e00277-17 (2017).
Braff, D., Shis, D. & Collins, J. J. Synthetic biology platform technologies for antimicrobial applications. Adv. Drug Deliv. Rev. 105, 35–43 (2016).
Ando, H., Lemire, S., Pires, D. P. & Lu, T. K. Engineering modular viral scaffolds for targeted bacterial population editing. Cell Syst. 1, 187–196 (2015).
Cerritelli, M. E., Wall, J. S., Simon, M. N., Conway, J. F. & Steven, A. C. Stoichiometry and domainal organization of the long tail-fiber of bacteriophage T4: a hinged viral adhesin. J. Mol. Biol. 260, 767–780 (1996).
Montag, D., Hashemolhosseini, S. & Henning, U. Receptor-recognizing proteins of T-even type bacteriophages. J. Mol. Biol. 216, 327–334 (1990).
Finn, R. D. et al. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res. 44, D279–285 (2016).
The UniProt Consortium UniProt: the universal protein knowledgebase. Nucleic Acids Res. 45, D158–D169 (2017).
Büttner, C. R., Wu, Y., Maxwell, K. L. & Davidson, A. R. Baseplate assembly of phage Mu: Defining the conserved core components of contractile-tailed phages and related bacterial systems. Proc. Natl Acad. Sci. USA 113, 10174–10179 (2016).
Howe, M. M., O’Day, K. J. & Schultz, D. W. Isolation of mutations defining five new cistrons essential for development of bacteriophage Mu. Virology 93, 303–319 (1979).
Holm, L. & Laakso, L. M. Dali server update. Nucleic Acids Res. 44, W351–W355 (2016).
Gibrat, J.-F., Madej, T. & Bryant, S. H. Surprising similarities in structure comparison. Curr. Opin. Struct. Biol. 6, 377–385 (1996).
Chen, M. et al. Inducible prophage mutant of Escherichia coli can lyse new host and the key sites of receptor recognition identification. Front. Microbiol. 8, 147 (2017).
Tetart, F., Repoila, F., Monod, C. & Krisch, H. M. Bacteriophage T4 host range is expanded by duplications of a small domain of the tail fiber adhesin. J. Mol. Biol. 258, 726–731 (1996).
Jakhetia, R. & Verma, N. K. Identification and molecular characterisation of a novel Mu-like bacteriophage, SfMu, of Shigella flexneri. PLoS ONE 10, e0124053 (2015).
Sandulache, R., Prehm, P., Expert, D., Toussaint, A. & Kamp, D. The cell wall receptor for bacteriophage Mu G(-) in Erwinia and Escherichia coli C. FEMS Microbiol. Lett. 28, 307–310 (1985).
Howe, M. M. Prophage deletion mapping of bacteriophage Mu-1. Virology 54, 93–101 (1973).
Pawluk, A. et al. Disabling a type I-E CRISPR-Cas nuclease with a bacteriophage-encoded anti-CRISPR protein. mBio 8, e01751–01717 (2017).
Abzalimov, R. R. et al. Studies of pH-dependent self-association of a recombinant form of arylsulfatase A with electrospray ionization mass spectrometry and size-exclusion chromatography. Anal. Chem. 85, 1591–1596 (2013).
Hendrix, R. & Duda, R. Bacteriophage lambda PaPa: not the mother of all lambda phages. Science 258, 1145–1148 (1992).
Riede, I., Drexler, K., Schwarz, H. & Henning, U. T-even-type bacteriophages use an adhesin for recognition of cellular receptors. J. Mol. Biol. 194, 23–30 (1987).
Dunne, M. et al. Salmonella phage S16 tail fiber adhesin features a rare polyglycine rich domain for host recognition. Structure 26, 1573–1582 (2018).
Montag, D. & Henning, U. An open reading frame in the Escherichia coli bacteriophage lambda genome encodes a protein that functions in assembly of the long tail fibers of bacteriophage T4. J. Bacteriol. 169, 5884–5886 (1987).
Tao, Y., Strelkov, S. V., Mesyanzhinov, V. V. & Rossmann, M. G. Structure of bacteriophage T4 fibritin: a segmented coiled coil and the role of the C-terminal domain. Structure 5, 789–798 (1997).
Schulz, E. C. et al. Crystal structure of an intramolecular chaperone mediating triple-beta-helix folding. Nat. Struct. Mol. Biol. 17, 210–215 (2010).
Marti, R. et al. Long tail fibres of the novel broad-host-range T-even bacteriophage S16 specifically recognize Salmonella OmpC. Mol. Microbiol. 87, 818–834 (2013).
Garcia-Doval, C. et al. Structure of the receptor-binding carboxy-terminal domain of the bacteriophage T5 L-shaped tail fibre with and without its intra-molecular chaperone. Viruses 7, 6424–6440 (2015).
Miroshnikov, K. A., Marusich, E. I., Cerritelli, M. E., Cheng, N., Hyde, C. C., Steven, A. C. & Mesyanzhinov, V. V. Engineering trimeric fibrous proteins based on bacteriophage T4 adhesins. Protein Eng. 11, 329–332 (1998).
Gorelik, M., Stanger, K. & Davidson, A. R. A conserved residue in the yeast Bem1p SH3 domain maintains the high level of binding specificity required for function. J. Biol. Chem. 286, 19470–19477 (2011).
Stollar, E. J. et al. Structural, functional, and bioinformatic studies demonstrate the crucial role of an extended peptide binding site for the SH3 domain of yeast Abp1p. J. Biol. Chem. 284, 26918–26927 (2009).
Demers, J. P. & Mittermaier, A. Binding mechanism of an SH3 domain studied by NMR and ITC. J. Am. Chem. Soc. 131, 4355–4367 (2009).
Zeng, D., Bhatt, V. S., Shen, Q. & Cho, J. H. Kinetic insights into the inding between the nSH3 domain of CrkII and proline-rich motifs in cAbl. Biophys. J. 111, 1843–1853 (2016).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D 67, 355–367 (2011).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D 66, 213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr D 60, 2126–2132 (2004).
Thaipisuttikul, I. et al. A divergent Pseudomonas aeruginosa palmitoyltransferase essential for cystic fibrosis-specific lipid A. Mol. Microbiol. 91, 158–174 (2014).
Acknowledgements
This work was supported by funding from the Canadian Institutes of Health Research to A.R.D. (Operating grant MOP-115039 and Foundation grant FDN-15427). S.T. was supported by a grant from Gunma University Medical Innovation Project and in part by the Cooperative Research Program of the Institute for Protein Research, Osaka University, CR-18-54. Diffraction data were collected at the Osaka University beamline BL44XU at SPring-8, Japan (Proposal nos. 2013B6500, 2014A6500 and 2014B6500). We thank P. Leiman and K. Maxwell for useful discussions of the manuscript.
Author information
Authors and Affiliations
Contributions
O.I.N. designed experiments, performed bioinformatic analysis, performed experiments, analysed data and wrote the manuscript. K.S. purified and obtained crystals of the TfibMu:TfaMu complex. S.T. supervised these experiments and helped in writing the manuscript. E.Y. and A.N. determined the TfibMu:TfaMu complex structure; T.I. performed the final refinement of the structure. C.R.B. obtained liquid chromatography–tandem mass spectrometry results of CsCl-purified wild-type phage Mu and created baseplate wedge constructs. A.R.D. supervised experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21, Supplementary Tables 1–3 and Supplementary References.
Rights and permissions
About this article
Cite this article
North, O.I., Sakai, K., Yamashita, E. et al. Phage tail fibre assembly proteins employ a modular structure to drive the correct folding of diverse fibres. Nat Microbiol 4, 1645–1653 (2019). https://doi.org/10.1038/s41564-019-0477-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0477-7
This article is cited by
-
Bacteriophages in nature: recent advances in research tools and diverse environmental and biotechnological applications
Environmental Science and Pollution Research (2024)
-
High-resolution cryo-EM structure of the Pseudomonas bacteriophage E217
Nature Communications (2023)
-
Distantly related Alteromonas bacteriophages share tail fibers exhibiting properties of transient chaperone caps
Nature Communications (2023)
-
Isolation, Characterization, and Genome Sequence Analysis of a Novel Lytic Phage, Xoo-sp15 Infecting Xanthomonas oryzae pv. oryzae
Current Microbiology (2021)