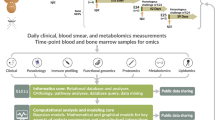
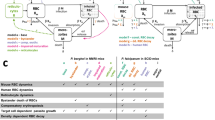
Abstract
During infection, increasing pathogen load stimulates both protective and harmful aspects of the host response. The dynamics of this interaction are hard to quantify in humans, but doing so could improve understanding of the mechanisms of disease and protection. We sought to model the contributions of the parasite multiplication rate and host response to observed parasite load in individual subjects infected with Plasmodium falciparum malaria, using only data obtained at the time of clinical presentation, and then to identify their mechanistic correlates. We predicted higher parasite multiplication rates and lower host responsiveness in cases of severe malaria, with severe anaemia being more insidious than cerebral malaria. We predicted that parasite-growth inhibition was associated with platelet consumption, lower expression of CXCL10 and type 1 interferon-associated genes, but increased cathepsin G and matrix metallopeptidase 9 expression. We found that cathepsin G and matrix metallopeptidase 9 directly inhibit parasite invasion into erythrocytes. The parasite multiplication rate was associated with host iron availability and higher complement factor H levels, lower expression of gametocyte-associated genes but higher expression of translation-associated genes in the parasite. Our findings demonstrate the potential of using explicit modelling of pathogen load dynamics to deepen understanding of host–pathogen interactions and identify mechanistic correlates of protection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Estimates of parameters determining within-host dynamics in the malariatherapy dataset were obtained from ref. 4, whose corresponding author may be contacted at klaus.dietz@uni-tuebingen.de. RNA-seq data have been deposited in the ArrayExpress database at EMBL-EBI (www.ebi.ac.uk/arrayexpress) under the accession number E-MTAB-6413. Individual subject-level data is available within the paper and its Supplementary information files. All other data that support the findings of this study are available from the corresponding author on reasonable request.
Code availability
The source code for the model simulating Gambian child subjects and examples of its use are presented as Supplementary Code library file and Supplementary Code example file.
References
Plotkin, S. A. Complex correlates of protection after vaccination. Clin. Infect. Dis. 56, 1458–1465 (2013).
Zumla, A. et al. Host-directed therapies for infectious diseases: current status, recent progress, and future prospects. Lancet Infect. Dis. 16, e47–e63 (2016).
Cunnington, A. J. The importance of pathogen load. PLoS Pathog. 11, e1004563 (2015).
Dietz, K., Raddatz, G. & Molineaux, L. Mathematical model of the first wave of Plasmodium falciparum asexual parasitemia in non-immune and vaccinated individuals. Am. J. Trop. Med. Hyg. 75, 46–55 (2006).
Kumaratilake, L. M. et al. A synthetic tumor necrosis factor-α agonist peptide enhances human polymorphonuclear leukocyte-mediated killing of Plasmodium falciparum in vitro and suppresses Plasmodium chabaudi infection in mice. J. Clin. Invest. 95, 2315–2323 (1995).
Kumaratilake, L. M., Ferrante, A. & Rzepczyk, C. M. Tumor necrosis factor enhances neutrophil-mediated killing of Plasmodium falciparum. Infect. Immun. 58, 788–793 (1990).
Smith, T. et al. Relationships between Plasmodium falciparum infection and morbidity in a highly endemic area. Parasitology 109, 539–549 (1994).
Crompton, P. D. et al. Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu. Rev. Immunol. 32, 157–187 (2014).
Okebe, J. et al. School-based countrywide seroprevalence survey reveals spatial heterogeneity in malaria transmission in The Gambia. PLoS ONE 9, e110926 (2014).
Cunnington, A. J., Walther, M. & Riley, E. M. Piecing together the puzzle of severe malaria. Sci. Transl. Med. 5, 211ps218 (2013).
Cunnington, A. J., Bretscher, M. T., Nogaro, S. I., Riley, E. M. & Walther, M. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J. Infect. 67, 220–230 (2013).
Hendriksen, I. C. et al. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J. Infect. Dis. 207, 351–361 (2013).
Kurtzhals, J. A. et al. Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351, 1768–1772 (1998).
Perkins, D. J. et al. Severe malarial anemia: innate immunity and pathogenesis. Int. J. Biol. Sci. 7, 1427–1442 (2011).
Cunnington, A. J., Riley, E. M. & Walther, M. Stuck in a rut? Reconsidering the role of parasite sequestration in severe malaria syndromes. Trends Parasitol. 29, 585–592 (2013).
Kho, S. et al. Platelets kill circulating parasites of all major Plasmodium species in human malaria. Blood 132, 1332–1344 (2018).
McMorran, B. J. et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 323, 797–800 (2009).
Cserti-Gazdewich, C. M. et al. Inter-relationships of cardinal features and outcomes of symptomatic pediatric Plasmodium falciparum malaria in 1,933 children in Kampala, Uganda. Am. J. Trop. Med. Hyg. 88, 747–756 (2013).
Lee, H. J. et al. Integrated pathogen load and dual transcriptome analysis of systemic host-pathogen interactions in severe malaria. Sci. Transl. Med. 10, eaar3619 (2018).
Arthur, J. S. & Ley, S. C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692 (2013).
Manning, B. D. & Toker, A. AKT/PKB signaling: navigating the network. Cell 169, 381–405 (2017).
Spaulding, E. et al. STING-licensed macrophages prime type I IFN production by plasmacytoid dendritic cells in the bone marrow during severe Plasmodium yoelii malaria. PLoS Pathog. 12, e1005975 (2016).
Zander, R. A. et al. Type I interferons induce T regulatory 1 responses and restrict humoral immunity during experimental malaria. PLoS Pathog. 12, e1005945 (2016).
Haque, A. et al. Type I IFN signaling in CD8– DCs impairs Th1-dependent malaria immunity. J. Clin. Invest. 124, 2483–2496 (2014).
Haque, A. et al. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur. J. Immunol. 41, 2688–2698 (2011).
Montes de Oca, M. et al. Type I interferons regulate immune responses in humans with blood-stage Plasmodium falciparum infection. Cell Rep. 17, 399–412 (2016).
Ioannidis, L. J. et al. Monocyte- and neutrophil-derived CXCL10 impairs efficient control of blood-stage malaria infection and promotes severe disease. J. Immunol. 196, 1227–1238 (2016).
Griffin, J. T. et al. Gradual acquisition of immunity to severe malaria with increasing exposure. Proc. R. Soc. B 282, 20142657 (2015).
Goncalves, B. P. et al. Parasite burden and severity of malaria in Tanzanian children. N. Engl. J. Med. 370, 1799–1808 (2014).
Cowland, J. B. & Borregaard, N. Granulopoiesis and granules of human neutrophils. Immunol. Rev. 273, 11–28 (2016).
Weksler, B. B., Jaffe, E. A., Brower, M. S. & Cole, O. F. Human leukocyte cathepsin G and elastase specifically suppress thrombin-induced prostacyclin production in human endothelial cells. Blood 74, 1627–1634 (1989).
D’Alessandro, S., Basilico, N. & Prato, M. Effects of Plasmodium falciparum-infected erythrocytes on matrix metalloproteinase-9 regulation in human microvascular endothelial cells. Asian Pac. J. Trop. Med. 6, 195–199 (2013).
Binks, R. H. & Conway, D. J. The major allelic dimorphisms in four Plasmodium falciparum merozoite proteins are not associated with alternative pathways of erythrocyte invasion. Mol. Biochem. Parasitol. 103, 123–127 (1999).
Bykowska, K., Duk, M., Kusnierz-Alejska, G., Kopec, M. & Lisowska, E. Degradation of human erythrocyte surface components by human neutrophil elastase and cathepsin G: preferential digestion of glycophorins. Br. J. Haematol. 84, 736–742 (1993).
Satchwell, T. J. Erythrocyte invasion receptors for Plasmodium falciparum: new and old. Transfus. Med. 26, 77–88 (2016).
Crosnier, C. et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 (2011).
Leffler, E. M. et al. Resistance to malaria through structural variation of red blood cell invasion receptors. Science 356, eaam6393 (2017).
Pasvol, G., Wainscoat, J. S. & Weatherall, D. J. Erythrocytes deficiency in glycophorin resist invasion by the malarial parasite Plasmodium falciparum. Nature 297, 64–66 (1982).
Clark, M. A. et al. Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum. Nat. Commun. 5, 4446 (2014).
Rosa, T. F. et al. The Plasmodium falciparum blood stages acquire factor H family proteins to evade destruction by human complement. Cell. Microbiol. 18, 573–590 (2016).
Kennedy, A. T. et al. Recruitment of factor H as a novel complement evasion strategy for blood-stage Plasmodium falciparum infection. J. Immunol. 196, 1239–1248 (2016).
Eriksson, U. K., van Bodegom, D., May, L., Boef, A. G. & Westendorp, R. G. Low C-reactive protein levels in a traditional West-African population living in a malaria endemic area. PLoS ONE 8, e70076 (2013).
Gwamaka, M. et al. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin. Infect. Dis. 54, 1137–1144 (2012).
Pasricha, S. R. et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci. Transl. Med. 6, 235re233 (2014).
Parente, R., Clark, S. J., Inforzato, A. & Day, A. J. Complement factor H in host defense and immune evasion. Cell. Mol. Life Sci. 74, 1605–1624 (2017).
van Beek, A. E. et al. Complement factor H levels associate with Plasmodium falciparum malaria susceptibility and severity. Open Forum Infect. Dis. 5, ofy166 (2018).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinform. 9, 559 (2008).
Zhang, M. et al. Uncovering the essential genes of the human malaria parasite Plasmodium falciparum by saturation mutagenesis. Science 360, eaap7847 (2018).
Lopez-Barragan, M. J. et al. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genom. 12, 587 (2011).
Josling, G. A. & Llinas, M. Sexual development in Plasmodium parasites: knowing when it’s time to commit. Nat. Rev. Microbiol. 13, 573–587 (2015).
Walther, M. et al. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in Gambian children. PLoS Pathog. 8, e1002579 (2012).
Walther, M. et al. Distinct roles for FOXP3+ and FOXP3− CD4+ T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 5, e1000364 (2009).
Marsh, K. et al. Indicators of life-threatening malaria in African children. N. Engl. J. Med. 332, 1399–1404 (1995).
R Core Development Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org (2014).
Wood, S. N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. B 73, 3–36 (2011).
Dondorp, A. M. et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2, e204 (2005).
Adetifa, I. M. et al. Haematological values from a Gambian cohort–possible reference range for a West African population. Int. J. Lab. Hematol. 31, 615–622 (2009).
Maier, A. G. & Rug, M. In vitro culturing Plasmodium falciparum erythrocytic stages. Methods Mol. Biol. 923, 3–15 (2013).
Trager, W. & Jensen, J. B. Human malaria parasites in continuous culture. Science 193, 673–675 (1976).
Dluzewski, A. R., Ling, I. T., Rangachari, K., Bates, P. A. & Wilson, R. J. A simple method for isolating viable mature parasites of Plasmodium falciparum from cultures. Trans. R. Soc. Trop. Med. Hyg. 78, 622–624 (1984).
Ribaut, C. et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 7, 45 (2008).
Malleret, B. et al. A rapid and robust tri-color flow cytometry assay for monitoring malaria parasite development. Sci. Rep. 1, 118 (2011).
Pouw, R. B. et al. Complement factor H-related protein 3 serum levels are low compared to factor H and mainly determined by gene copy number variation in CFHR3. PLoS ONE 11, e0152164 (2016).
Acknowledgements
We are grateful to K. Dietz for providing the original data and parameter estimates from malariatherapy patients, and his model, and to the St. Mary’s NHLI FACS core facility and Y. Guo for support and instrumentation. This work was supported by the Medical Research Council (MRC) UK via core funding to the malaria research programme at the MRC Unit, The Gambia; the UK MRC and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement that is also part of the EDCTP2 program supported by the European Union (MR/L006529/1 to A.J.C.); a Wellcome Trust Value In People Award to A.J.C. and the European Union’s seventh Framework program under EC-GA no. 279185 (EUCLIDS; www.euclids-project.eu).
Author information
Authors and Affiliations
Contributions
A.J.C., A.G., A.E.v.B., F.F., D.J.C., D.N. and M.W. collected the data used in the study. A.J.C., E.M.R., M.T.B., M.W. and D.J.C. designed the study. A.J.C. and M.T.B. developed the mathematical model. A.J.C., M.T.B., H.J.L., F.F., T.D.O. and A.E.v.B. analysed the data. D.W., T.W.K, D.N., U.D., E.M.R., M.L., L.J.C., A.G., D.J.C. and A.J.C. supervised aspects of the project. All authors contributed to the interpretation of the results and drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–6, Supplementary Tables 1–12, Supplementary dataset legends.
Supplementary Data 1
Data from the Gambian children with malaria
Supplementary Code Example File
R example file for supplementary code for the mathematical model.
Supplementary Code Explanation File
README file explaining supplementary code.
Supplementary Code Library File
R library file with supplementary code for the mathematical model.
Rights and permissions
About this article
Cite this article
Georgiadou, A., Lee, H.J., Walther, M. et al. Modelling pathogen load dynamics to elucidate mechanistic determinants of host–Plasmodium falciparum interactions. Nat Microbiol 4, 1592–1602 (2019). https://doi.org/10.1038/s41564-019-0474-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0474-x
This article is cited by
-
Modelling upper respiratory viral load dynamics of SARS-CoV-2
BMC Medicine (2022)
-
Malaria parasites both repress host CXCL10 and use it as a cue for growth acceleration
Nature Communications (2021)
-
Intrinsic multiplication rate variation and plasticity of human blood stage malaria parasites
Communications Biology (2020)
-
Leveraging Computational Modeling to Understand Infectious Diseases
Current Pathobiology Reports (2020)