Abstract
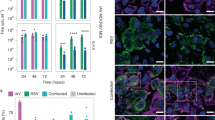
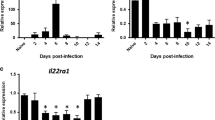
Epidemiological observations and animal models have long shown synergy between influenza virus infections and bacterial infections. Influenza virus infection leads to an increase in both the susceptibility to secondary bacterial infections and the severity of the bacterial infections, primarily pneumonias caused by Streptococcus pneumoniae or Staphylococcus aureus. We show that, in addition to the widely described immune modulation and tissue-remodelling mechanisms of bacterial–viral synergy, the virus interacts directly with the bacterial surface. Similar to the recent observation of direct interactions between enteric bacteria and enteric viruses, we observed a direct interaction between influenza virus on the surface of Gram-positive, S. pneumoniae and S. aureus, and Gram-negative, Moraxella catarrhalis and non-typeable Haemophilus influenzae, bacterial colonizers and pathogens in the respiratory tract. Pre-incubation of influenza virus with bacteria, followed by the removal of unbound virus, increased bacterial adherence to respiratory epithelial cells in culture. This result was recapitulated in vivo, with higher bacterial burdens in murine tissues when infected with pneumococci pre-incubated with influenza virus versus control bacteria without virus. These observations support an additional mechanism of bacteria–influenza virus synergy at the earliest steps of pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support these findings are available from J. Rosch on request.
References
Kuss, S. K. et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334, 249–252 (2011).
Erickson, A. K. et al. Bacteria facilitate enteric virus co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe 23, 77–88 (2018).
Robinson, C. M., Jesudhasan, P. R. & Pfeiffer, J. K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15, 36–46 (2014).
Berger, A. K., Yi, H., Kearns, D. B. & Mainou, B. A. Bacteria and bacterial envelope components enhance mammalian reovirus thermostability. PLoS Pathog. 13, e1006768 (2017).
Pfeiffer, J. K. & Virgin, H. W. Viral immunity. Transkingdom control of viral infection and immunity in the mammalian intestine. Science 351, aad5872 (2016).
Karst, S. M. The influence of commensal bacteria on infection with enteric viruses. Nat. Rev. Microbiol. 14, 197–204 (2016).
Berger, A. K. & Mainou, B. A. Interactions between enteric bacteria and eukaryotic viruses impact the outcome of infection. Viruses 10, 19 (2018).
Falsey, A. R. et al. Bacterial complications of respiratory tract viral illness: a comprehensive evaluation. J. Infect. Dis. 208, 432–441 (2013).
McCullers, J. A. Do specific virus-bacteria pairings drive clinical outcomes of pneumonia? Clin. Microbiol. Infect. 19, 113–118 (2013).
McCullers, J. A. & Rehg, J. E. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186, 341–350 (2002).
McCullers, J. A. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat. Rev. Microbiol. 12, 252–262 (2014).
Ghoneim, H. E., Thomas, P. G. & McCullers, J. A. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 191, 1250–1259 (2013).
Mina, M. J., Klugman, K. P., Rosch, J. W. & McCullers, J. A. Live attenuated influenza virus increases pneumococcal translocation and persistence within the middle ear. J. Infect. Dis. 212, 195–201 (2015).
Smith, C. M. et al. Respiratory syncytial virus increases the virulence of Streptococcus pneumoniae by binding to penicillin binding protein 1a. A new paradigm in respiratory infection. Am. J. Respir. Crit. Care Med. 190, 196–207 (2014).
Hament, J. M. et al. Direct binding of respiratory syncytial virus to pneumococci: a phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr. Res. 58, 1198–1203 (2005).
Avadhanula, V., Wang, Y., Portner, A. & Adderson, E. Nontypeable Haemophilus influenzae and Streptococcus pneumoniae bind respiratory syncytial virus glycoprotein. J. Med. Microbiol. 56, 1133–1137 (2007).
Van Ewijk, B. E. et al. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr. Res. 61, 398–403 (2007).
Wu, N. H., Meng, F., Seitz, M., Valentin-Weigand, P. & Herrler, G. Sialic acid-dependent interactions between influenza viruses and Streptococcus suis affect the infection of porcine tracheal cells. J. Gen. Virol. 96, 2557–2568 (2015).
Wang, Y. et al. Capsular sialic acid of Streptococcus suis serotype 2 binds to swine influenza virus and enhances bacterial interactions with virus-infected tracheal epithelial cells. Infect. Immun. 81, 4498–4508 (2013).
Harding A. T., Heaton B. E., Dumm R. E. & Heaton N. S. Rationally designed influenza virus vaccines that are antigenically stable during growth in eggs. mBio 8, e00669-17 (2017).
Rudd J. M., Ashar H. K., Chow V. T. & Teluguakula N. Lethal Synergism between Influenza and Streptococcus pneumoniae. J. Infect. Pulm. Dis. https://doi.org/10.16966/2470-3176.114 (2016).
Edouard, S. et al. The nasopharyngeal microbiota in patients with viral respiratory tract infections is enriched in bacterial pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1725–1733 (2018).
Navne, J. E. et al. Nasopharyngeal bacterial carriage in young children in Greenland: a population at high risk of respiratory infections. Epidemiol. Infect. 144, 3226–3236 (2016).
Huber, V. C., Thomas, P. G. & McCullers, J. A. A multi-valent vaccine approach that elicits broad immunity within an influenza subtype. Vaccine 27, 1192–1200 (2009).
Rosch, J. W. et al. A live-attenuated pneumococcal vaccine elicits CD4+ T-cell dependent class switching and provides serotype independent protection against acute otitis media. EMBO Mol. Med. 6, 141–154 (2014).
Kash J. C. et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. mBio 2, e00172-11 (2011).
Speshock, J. L., Doyon-Reale, N., Rabah, R., Neely, M. N. & Roberts, P. C. Filamentous influenza A virus infection predisposes mice to fatal septicemia following superinfection with Streptococcus pneumoniae serotype 3. Infect. Immun. 75, 3102–3111 (2007).
Wolter, N. et al. High nasopharyngeal pneumococcal density, increased by viral coinfection, is associated with invasive pneumococcal pneumonia. J. Infect. Dis. 210, 1649–1657 (2014).
El Ahmer, O. R., Raza, M. W., Ogilvie, M. M., Weir, D. M. & Blackwell, C. C. Binding of bacteria to HEp-2 cells infected with influenza A virus. FEMS Immunol. Med. Microbiol. 23, 331–341 (1999).
Bandoro C. & Runstadler J. A. Bacterial lipopolysaccharide destabilizes influenza viruses. mSphere 2, e00267-17 (2017).
King, S. J., Hippe, K. R. & Weiser, J. N. Deglycosylation of human glycoconjugates by the sequential activities of exoglycosidases expressed by Streptococcus pneumoniae. Mol. Microbiol. 59, 961–974 (2006).
Heikkinen, T. & Chonmaitree, T. Importance of respiratory viruses in acute otitis media. Clin. Microbiol. Rev. 16, 230–241 (2003).
Chonmaitree, T. et al. Viral upper respiratory tract infection and otitis media complication in young children. Clin. Infect. Dis. 46, 815–823 (2008).
Wadowsky, R. M., Mietzner, S. M., Skoner, D. P., Doyle, W. J. & Fireman, P. Effect of experimental influenza A virus infection on isolation of Streptococcus pneumoniae and other aerobic bacteria from the oropharynges of allergic and nonallergic adult subjects. Infect. Immun. 63, 1153–1157 (1995).
Short, K. R. et al. Influenza virus induces bacterial and nonbacterial otitis media. J. Infect. Dis. 204, 1857–1865 (2011).
Kim, J. K. & Cho, J. H. Change of external auditory canal pH in acute otitis externa. Ann. Otol. Rhinol. Laryngol. 118, 769–772 (2009).
Weiser, J. N., Ferreira, D. M. & Paton, J. C. Streptococcus pneumoniae: transmission, colonization and invasion. Nat. Rev. Microbiol. 16, 355–367 (2018).
Zafar, M. A., Wang, Y., Hamaguchi, S. & Weiser, J. N. Host-to-host transmission of Streptococcus pneumoniae is driven by its inflammatory toxin, pneumolysin. Cell Host Microbe 21, 73–83 (2017).
Zafar, M. A., Kono, M., Wang, Y., Zangari, T. & Weiser, J. N. Infant mouse model for the study of shedding and transmission during Streptococcus pneumoniae monoinfection. Infect. Immun. 84, 2714–2722 (2016).
Grijalva, C. G. et al. The role of influenza and parainfluenza infections in nasopharyngeal pneumococcal acquisition among young children. Clin. Infect. Dis. 58, 1369–1376 (2014).
Harrison, A. et al. Genomic sequence of an otitis media isolate of nontypeable Haemophilus influenzae: comparative study with H. influenzae serotype d, strain KW20. J. Bacteriol. 187, 4627–4636 (2005).
Helminen, M. E. et al. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J. Infect. Dis. 170, 867–872 (1994).
Cline, T. D. et al. Increased pathogenicity of a reassortant 2009 pandemic H1N1 influenza virus containing an H5N1 hemagglutinin. J. Virol. 85, 12262–12270 (2011).
Reed, L. J. & Meunch, H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27, 493–497 (1938).
Acknowledgements
J.W.R. is supported by the National Institutes of Allergic and Infectious Diseases (NIAID) (grant nos. 1U01AI124302 and 1RO1AI110618). S.S.-C. is supported by the NIAID (grant no. HHSN272201400006C). J.W.R. and S.S.-C. are funded by the ALSAC. Images were acquired in the Cell and Tissue Imaging Center, which is supported by St. Jude and NCI P30 CA021765.
Author information
Authors and Affiliations
Contributions
H.M.R., V.A.M., A.I. and P.B. performed the experiments. H.M.R., S.S.-C. and J.W.R. conceived and designed the experiments, and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–3 and Supplementary Video legends.
Supplementary Video 1
3D rendering and rotation of SIM image of mRuby2–PR8 (red) on surface of S. pneumoniae stained with AF488-WGA (green). Representative from at least 10 bacterial–viral complexes.
Supplementary Video 2
3D rendering and rotation, and Z-stack slices of SIM image of mRuby2–PR8 (red) on surface of S. aureus stained with AF488-WGA (green). Representative from at least 10 bacterial-viral complexes.
Supplementary Video 3
3D rendering and rotation of SIM image of mRuby2–PR8 (red) on surface of M. catarrhalis stained with AF488-WGA (green). Representative from at least 10 bacterial–viral complexes.
Supplementary Video 4
3D rendering and rotation of SIM image of mRuby2–PR8 (red) on surface of H. influenzae stained with stained with MitoTracker DeepRed (false coloured to green). Representative from at least 10 bacterial–viral complexes.
Rights and permissions
About this article
Cite this article
Rowe, H.M., Meliopoulos, V.A., Iverson, A. et al. Direct interactions with influenza promote bacterial adherence during respiratory infections. Nat Microbiol 4, 1328–1336 (2019). https://doi.org/10.1038/s41564-019-0447-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-019-0447-0
This article is cited by
-
Comparison of the adherence of nontypeable haemophilus influenzae to lung epithelial cells
BMC Infectious Diseases (2024)
-
Risk factors, outcomes, and epidemiological and etiological study of hospitalized COVID-19 patients with bacterial co-infection and secondary infections
European Journal of Clinical Microbiology & Infectious Diseases (2024)
-
Synthesis, characterization, and photocatalytic antibacterial activities of porous Ce-doped TiO2 microspheres using pine pollen as novel biotemplates
Journal of Materials Science (2022)
-
The role of co-infections and secondary infections in patients with COVID-19
Pneumonia (2021)
-
Host factors facilitating SARS‐CoV‐2 virus infection and replication in the lungs
Cellular and Molecular Life Sciences (2021)