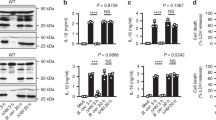
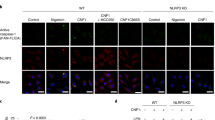
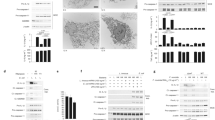
Abstract
Host recognition of microbial components is essential in mediating an effective immune response. Cytosolic bacteria must secure entry into the host cytoplasm to facilitate replication and, in doing so, liberate microbial ligands that activate cytosolic innate immune sensors and the inflammasome. Here, we identified a multicomponent enterotoxin, haemolysin BL (HBL), that engages activation of the inflammasome. This toxin is highly conserved among the human pathogen Bacillus cereus. The three subunits of HBL bind to the cell membrane in a linear order, forming a lytic pore and inducing activation of the NLRP3 inflammasome, secretion of interleukin-1β and interleukin-18, and pyroptosis. Mechanistically, the HBL-induced pore results in the efflux of potassium and triggers the activation of the NLRP3 inflammasome. Furthermore, HBL-producing B. cereus induces rapid inflammasome-mediated mortality. Pharmacological inhibition of the NLRP3 inflammasome using MCC950 prevents B. cereus-induced lethality. Overall, our results reveal that cytosolic sensing of a toxin is central to the innate immune recognition of infection. Therapeutic modulation of this pathway enhances host protection against deadly bacterial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are included in this published article along with its Supplementary Information files, and are available from the corresponding author upon request.
References
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Lamkanfi, M. & Dixit, V. M. Mechanisms and functions of inflammasomes. Cell 157, 1013–1022 (2014).
Rathinam, V. A. & Fitzgerald, K. A. Inflammasome complexes: emerging mechanisms and effector functions. Cell 165, 792–800 (2016).
Latz, E., Xiao, T. S. & Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 13, 397–411 (2013).
Man, S. M. & Kanneganti, T. D. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat. Rev. Immunol. 16, 7–21 (2016).
Man, S. M. et al. IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11–NLRP3 Inflammasomes. Cell 167, 382–396.e317 (2016).
Man, S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014).
Meunier, E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011).
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Amer, A. et al. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 (2006).
Liu, S., Moayeri, M. & Leppla, S. H. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol. 22, 317–325 (2014).
Dal Peraro, M. & van der Goot, F. G. Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 (2016).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Muñoz-Planillo, R., Franchi, L., Miller, L. S. & Núñez, G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183, 3942–3948 (2009).
Craven, R. R. et al. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS ONE 4, e7446 (2009).
Kebaier, C. et al. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J. Infect. Dis. 205, 807–817 (2012).
Zhang, X. et al. Enterohemorrhagic Escherichia coli specific enterohemolysin induced IL-1beta in human macrophages and EHEC-induced IL-1beta required activation of NLRP3 inflammasome. PLoS ONE 7, e50288 (2012).
Schaale, K. et al. Strain- and host species-specific inflammasome activation, IL-1β release, and cell death in macrophages infected with uropathogenic Escherichia coli. Mucosal Immunol. 9, 124 (2015).
Costa, A. et al. Activation of the NLRP3 inflammasome by group B streptococci. J. Immunol. 188, 1953–1960 (2012).
Whidbey, C. et al. A streptococcal lipid toxin induces membrane permeabilization and pyroptosis leading to fetal injury. EMBO Mol. Med. 7, 488–505 (2015).
Gupta, R. et al. RNA and beta-hemolysin of group B Streptococcus induce interleukin-1beta (IL-1beta) by activating NLRP3 inflammasomes in mouse macrophages. J. Biol. Chem. 289, 13701–13705 (2014).
Harder, J. et al. Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor. J. Immunol. 183, 5823–5829 (2009).
Keyel, P. A., Roth, R., Yokoyama, W. M., Heuser, J. E. & Salter, R. D. Reduction of streptolysin O (SLO) pore-forming activity enhances inflammasome activation. Toxins 5, 1105–1118 (2013).
Ozoren, N. et al. Distinct roles of TLR2 and the adaptor ASC in IL-1β/IL-18 secretion in response to Listeria monocytogenes. J. Immunol. 176, 4337–4342 (2006).
Hamon, M. A. & Cossart, P. K+ efflux is required for histone H3 dephosphorylation by Listeria monocytogenes listeriolysin O and other pore-forming toxins. Infect. Immun. 79, 2839–2846 (2011).
Toma, C. et al. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling. J. Immunol. 184, 5287–5297 (2010).
Higa, N. et al. Vibrio parahaemolyticus effector proteins suppress inflammasome activation by interfering with host autophagy signaling. PLoS Pathog. 9, e1003142 (2013).
Song, L. et al. A critical role for hemolysin in Vibrio fluvialis-induced IL-1beta secretion mediated by the NLRP3 inflammasome in macrophages. Front. Microbiol. 6, 510 (2015).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Man, S. M. & Kanneganti, T. D. Regulation of inflammasome activation. Immunol. Rev. 265, 6–21 (2015).
Bottone, E. J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 23, 382–398 (2010).
Beecher, D. J. & Wong, A. C. Improved purification and characterization of hemolysin BL, a hemolytic dermonecrotic vascular permeability factor from Bacillus cereus. Infect. Immun. 62, 980–986 (1994).
Heinrichs, J. H., Beecher, D. J., MacMillan, J. D. & Zilinskas, B. A. Molecular cloning and characterization of the hblA gene encoding the B component of hemolysin BL from Bacillus cereus. J. Bacteriol. 175, 6760–6766 (1993).
Ryan, P. A., Macmillan, J. D. & Zilinskas, B. A. Molecular cloning and characterization of the genes encoding the L1 and L2 components of hemolysin BL from Bacillus cereus. J. Bacteriol. 179, 2551–2556 (1997).
Sastalla, I. et al. The Bacillus cereus Hbl and Nhe tripartite enterotoxin components assemble sequentially on the surface of target cells and are not interchangeable. PLoS ONE 8, e76955 (2013).
Lund, T., De Buyser, M. L. & Granum, P. E. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38, 254–261 (2000).
Beecher, D. J. & Wong, A. C. Tripartite hemolysin BL from Bacillus cereus. Hemolytic analysis of component interactions and a model for its characteristic paradoxical zone phenomenon. J. Biol. Chem. 272, 233–239 (1997).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Hellmich, K. A. et al. Anthrax lethal factor cleaves mouse nlrp1b in both toxin-sensitive and toxin-resistant macrophages. PLoS ONE 7, e49741 (2012).
Moayeri, M. et al. Inflammasome sensor Nlrp1b-dependent resistance to anthrax is mediated by caspase-1, IL-1 signaling and neutrophil recruitment. PLoS Pathog. 6, e1001222 (2010).
Man, S. M. et al. Actin polymerization as a key innate immune effector mechanism to control Salmonella infection. Proc. Natl Acad. Sci. USA 111, 17588–17593 (2014).
Madegowda, M., Eswaramoorthy, S., Burley, S. K. & Swaminathan, S. X-ray crystal structure of the B component of hemolysin BL from Bacillus cereus. Proteins 71, 534–540 (2008).
Yohannan, S., Faham, S., Yang, D., Whitelegge, J. P. & Bowie, J. U. The evolution of transmembrane helix kinks and the structural diversity of G protein-coupled receptors. Proc. Natl Acad. Sci. USA 101, 959–963 (2004).
Cordes, F. S., Bright, J. N. & Sansom, M. S. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323, 951–960 (2002).
Jin, T. et al. The (beta)gamma subunits of G proteins gate a K(+) channel by pivoted bending of a transmembrane segment. Mol. Cell 10, 469–481 (2002).
Tieleman, D. P., Shrivastava, I. H., Ulmschneider, M. R. & Sansom, M. S. Proline-induced hinges in transmembrane helices: possible roles in ion channel gating. Proteins 44, 63–72 (2001).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Jorgensen, I., Zhang, Y., Krantz, B. A. & Miao, E. A. Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med. 213, 2113–2128 (2016).
Hayward, J. A., Mathur, A., Ngo, C. & Man, S. M. Cytosolic recognition of microbes and pathogens: inflammasomes in action. Microbiol. Mol. Biol. Rev. 82, e00015-18 (2018).
Perregaux, D. & Gabel, C. A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994).
Petrilli, V. et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 (2007).
Munoz-Planillo, R. et al. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 (2013).
Henry, T., Brotcke, A., Weiss, D. S., Thompson, L. J. & Monack, D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
Hara, H. et al. Dependency of caspase-1 activation induced in macrophages by Listeria monocytogenes on cytolysin, listeriolysin O, after evasion from phagosome into the cytoplasm. J. Immunol. 180, 7859–7868 (2008).
Ehling-Schulz, M., Fricker, M. & Scherer, S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol. Nutr. Food. Res. 48, 479–487 (2004).
Man, S. M. et al. Differential roles of caspase-1 and caspase-11 in infection and inflammation. Sci. Rep. 7, 45126 (2017).
Tate, M. D. et al. Reassessing the role of the NLRP3 inflammasome during pathogenic influenza A virus infection via temporal inhibition. Sci. Rep. 6, 27912 (2016).
Primiano, M. J. et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J. Immunol. 197, 2421–2433 (2016).
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 66, 1037–1046 (2017).
Chen, W. et al. Specific inhibition of NLRP3 in chikungunya disease reveals a role for inflammasomes in alphavirus-induced inflammation. Nat. Microbiol. 2, 1435–1445 (2017).
Moayeri, M. et al. Small-molecule inhibitors of lethal factor protease activity protect against anthrax infection. Antimicrob. Agents Chemother. 57, 4139–4145 (2013).
Leysath, C. E. et al. Mouse monoclonal antibodies to anthrax edema factor protect against infection. Infect. Immun. 79, 4609–4616 (2011).
Lund, T. & Granum, P. E. Characterisation of a non-haemolytic enterotoxin complex from Bacillus cereus isolated after a foodborne outbreak. FEMS Microbiol. Lett. 141, 151–156 (1996).
Ganash, M. et al. Structure of the NheA component of the Nhe toxin from Bacillus cereus: implications for function. PLoS ONE 8, e74748 (2013).
Haug, T. M. et al. Formation of very large conductance channels by Bacillus cereus Nhe in Vero and GH(4) cells identifies NheA + B as the inherent pore-forming structure. J. Membr. Biol. 237, 1–11 (2010).
Jones, J. W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl Acad. Sci. USA 107, 9771–9776 (2010).
Kuida, K. et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science 267, 2000–2003 (1995).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
Kovarova, M. et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J. Immunol. 189, 2006–2016 (2012).
Dietrich, R., Moravek, M., Burk, C., Granum, P. E. & Martlbauer, E. Production and characterization of antibodies against each of the three subunits of the Bacillus cereus nonhemolytic enterotoxin complex. Appl. Environ. Microbiol. 71, 8214–8220 (2005).
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
Snider, C., Jayasinghe, S., Hristova, K. & White, S. H. MPEx: a tool for exploring membrane proteins. Protein Sci. 18, 2624–2628 (2009).
Gautier, R., Douguet, D., Antonny, B. & Drin, G. HELIQUEST: a web server to screen sequences with specific alpha-helical properties. Bioinformatics 24, 2101–2102 (2008).
Acknowledgements
The authors would like to thank V. M. Dixit (Genentech, USA), K. Schroder (Institute of Molecular Bioscience, Australia), P. Broz (University of Lausanne, Switzerland), J. Ng (Westmead Hospital, Australia), A. Rice (The Canberra Hospital, Australia) and J. Bates (Department of Health Queensland, Australia) for reagents. They also thank B. Quah (ANU, Australia), C. Gillespie (ANU, Australia), I. Sastalla (National Institutes of Health, USA), M. Rug (Centre for Advanced Microscopy, ANU, Australia), J. Lee (Centre for Advanced Microscopy, ANU, Australia), C. O’Brien (The Canberra Hospital, Australia) and D. Gordon (ANU, Australia) for assistance. A.M. is supported by a John Curtin School of Medical Research International Ph.D. scholarship. S.H.L. is supported, in part, by the Intramural Program of the National Institute of Allergy and Infectious Diseases, NIH, USA. N.O.K. is supported by a Career Development Fellowship from the Cancer Institute NSW (15/CDF/1-11). S.M.M. is supported by the Australian National University, The Gretel and Gordon Bootes Medical Research Foundation, and the National Health and Medical Research Council of Australia (under Project Grants APP1141504 and APP1146864) and the R.G. Menzies Early Career Fellowship (APP1091544).
Author information
Authors and Affiliations
Contributions
A.M. and S.M.M. conceptualized the study. A.M., S.F., J.A.H., C.N., D.F., I.I.A., J.D.P. and N.O.K. performed the experiments. A.M., S.F., J.A.H., C.N., D.F. and N.O.K. conducted the analyses, and A.M. and S.M.M. wrote the manuscript. S.M.M. acquired the funding, and I.I.A., J.D.P., K.S., E.M., A.A.B.R., G.B., E.M.F. and S.H.L. provided resources and intellectual input. S.M.M. provided overall supervision, and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
I.I.A. is Director of Lipotek, a niche biotech company with a focus on liposome technology. I.I.A. and J.D.P. are shareholders of Lipotek. A.A.B.R. is a named inventor on inflammasome inhibitor patents (WO2017140778 and WO2016131098). All other authors have no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–11, Supplementary Blots, Supplementary Tables 1 and 2, and Supplementary References.
Rights and permissions
About this article
Cite this article
Mathur, A., Feng, S., Hayward, J.A. et al. A multicomponent toxin from Bacillus cereus incites inflammation and shapes host outcome via the NLRP3 inflammasome. Nat Microbiol 4, 362–374 (2019). https://doi.org/10.1038/s41564-018-0318-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0318-0
This article is cited by
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
Bacillus cereus cereolysin O induces pyroptosis in an undecapeptide-dependent manner
Cell Death Discovery (2024)
-
Bacillus cereus extracellular vesicles act as shuttles for biologically active multicomponent enterotoxins
Cell Communication and Signaling (2023)
-
Lactobacillus rhamnosus GR-1 attenuates foodborne Bacillus cereus-induced NLRP3 inflammasome activity in bovine mammary epithelial cells by protecting intercellular tight junctions
Journal of Animal Science and Biotechnology (2022)
-
Bacillus cereus cytotoxin K triggers gasdermin D-dependent pyroptosis
Cell Death Discovery (2022)