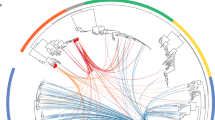
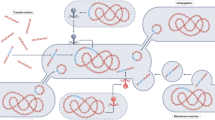
Abstract
The human gut microbiota has adapted to the presence of antimicrobial peptides (AMPs), which are ancient components of immune defence. Despite its medical importance, it has remained unclear whether AMP resistance genes in the gut microbiome are available for genetic exchange between bacterial species. Here, we show that AMP resistance and antibiotic resistance genes differ in their mobilization patterns and functional compatibilities with new bacterial hosts. First, whereas AMP resistance genes are widespread in the gut microbiome, their rate of horizontal transfer is lower than that of antibiotic resistance genes. Second, gut microbiota culturing and functional metagenomics have revealed that AMP resistance genes originating from phylogenetically distant bacteria have only a limited potential to confer resistance in Escherichia coli, an intrinsically susceptible species. Taken together, functional compatibility with the new bacterial host emerges as a key factor limiting the genetic exchange of AMP resistance genes. Finally, our results suggest that AMPs induce highly specific changes in the composition of the human microbiota, with implications for disease risks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The GenBank accession nos. for the PacBio sequencing data are MH883365–MH883616. 16S rRNA sequencing reads are available from the Sequence Read Archive (SRA) (BioProject PRJNA494380). All data generated or analysed during this study are included in this article and its Supplementary Information. For each figure, the availability of the analysed data is indicated in the legend. All accession numbers with information on the associated samples are provided as Supplementary Data.
References
Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. An ecological and evolutionary perspective on human–microbe mutualism and disease. Nature 449, 811–818 (2007).
Nicholson, J. K. et al. Host–gut microbiota metabolic interactions. Science 336, 1262–1267 (2012).
Littman, D. R. & Pamer, E. G. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10, 311–323 (2011).
Round, J. L. & Mazmanian, S. K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9, 600–600 (2009).
Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 415, 389–395 (2002).
Peschel, A. & Sahl, H. G. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4, 529–536 (2006).
Hancock, R. & Patrzykat, A. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Target Infect. Disord. 2, 79–83 (2002).
Hancock, R. E. W. & Sahl, H.-G. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557 (2006).
Kubicek-Sutherland, J. Z. et al. Antimicrobial peptide exposure selects for Staphylococcus aureus resistance to human defence peptides. J. Antimicrob. Chemother. 72, 115–127 (2017).
Andersson, D. I., Hughes, D. & Kubicek-Sutherland, J. Z. Mechanisms and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 26, 43–57 (2016).
Sommer, M. O. A., Dantas, G. & Church, G. M. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science 325, 1128–1131 (2009).
Cullen, T. W. et al. Gut microbiota. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. Science 347, 170–175 (2015).
Liu, Y.-Y. et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168 (2016).
Chen, L. et al. Newly identified colistin resistance genes, mcr-4 and mcr-5, from upper and lower alimentary tract of pigs and poultry in China. PLoS ONE 13, e0193957 (2018).
Brito, I. L. et al. Mobile genes in the human microbiome are structured from global to individual scales. Nature 535, 435–439 (2016).
Jain, R., Rivera, M. C. & Lake, J. A. Horizontal gene transfer among genomes: the complexity hypothesis. Proc. Natl Acad. Sci. USA 96, 3801–3806 (1999).
Cohen, O., Gophna, U. & Pupko, T. The complexity hypothesis revisited: connectivity rather than function constitutes a barrier to horizontal gene transfer. Mol. Biol. Evol. 28, 1481–1489 (2011).
Forsberg, K. J. et al. Bacterial phylogeny structures soil resistomes across habitats. Nature 509, 612–616 (2014).
van der Helm, E. et al. Rapid resistome mapping using nanopore sequencing. Nucleic Acids Res. 45, e61 (2017).
Pehrsson, E. C. et al. Interconnected microbiomes and resistomes in low-income human habitats. Nature 533, 212–216 (2016).
Kaye, K. S., Pogue, J. M., Tran, T. B., Nation, R. L. & Li, J. Agents of last resort. Infect. Dis. Clin. North Am. 30, 391–414 (2016).
Zhi, C., Lv, L., Yu, L.-F., Doi, Y. & Liu, J.-H. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 16, 292–293 (2016).
Dürr, U. H. N., Sudheendra, U. S. & Ramamoorthy, A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta 1758, 1408–1425 (2006).
Methé, B. A. et al. A framework for human microbiome research. Nature 486, 215–221 (2012).
Rettedal, E. A., Gumpert, H. & Sommer, M. O. A. Cultivation-based multiplex phenotyping of human gut microbiota allows targeted recovery of previously uncultured bacteria. Nat. Commun. 5, 4714 (2014).
Rowan, F., Docherty, N. & Murphy, M. Desulfovibrio bacterial species are increased in ulcerative colitis. Dis. Colon 53, 1530–1536 (2010).
Wehkamp, J. et al. Inducible and constitutive β-defensins are differentially expressed in Crohn’s disease and ulcerative colitis. Inflamm. Bowel Dis. 9, 215–223 (2003).
Lopetuso, L. R., Scaldaferri, F., Petito, V. & Gasbarrini, A. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 5, 23 (2013).
Coats, S. R., To, T. T., Jain, S., Braham, P. H. & Darveau, R. P. Porphyromonas gingivalis resistance to polymyxin B is determined by the lipid A 4′-phosphatase, PGN_0524. Int. J. Oral Sci. 1, 126–135 (2009).
Ingram, B. O., Masoudi, A. & Raetz, C. R. H. Escherichia coli mutants that synthesize dephosphorylated lipid A molecules. Biochemistry 49, 8325–8337 (2010).
Porse, A., Schou, T. S., Munck, C., Ellabaan, M. M. H. & Sommer, M. O. A. Biochemical mechanisms determine the functional compatibility of heterologous genes. Nat. Commun. 9, 522 (2018).
Kong, Q. et al. Phosphate groups of lipid A are essential for Salmonella enterica serovar Typhimurium virulence and affect innate and adaptive immunity. Infect. Immun. 80, 3215–3224 (2012).
Wang, R. et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. N at. Commu n. 9, 1179 (2018).
Huerta-Cepas, J., Serra, F. & Bork, P. ETE 3: Reconstruction, analysis and visualization of phylogenomic data. Mol. Biol. Evol. 33, 1635–1638 (2016).
McArthur, A. G. et al. The comprehensive antibiotic resistance database. Antimicrob. Agents Chemother. 57, 3348–3357 (2013).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Lagesen, K. et al. RNAmmer: consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 35, 3100–3108 (2007).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
O’Leary, N. A. et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion and functional annotation. Nucleic Acids Res. 44, D733–D745 (2016).
Orlek, A. et al. A curated dataset of complete Enterobacteriaceae plasmids compiled from the NCBI nucleotide database. Data Brief 12, 423–426 (2017).
Smillie, C. S. et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 480, 241–244 (2011).
Jiang, X. et al. Comprehensive analysis of mobile genetic elements in the gut microbiome reveals a phylum-level niche-adaptive gene pool. Preprint at https://www.biorxiv.org/content/early/2017/11/06/214213 (2017).
Szybalski, W. Genetic studies on microbial cross resistance to toxic agents. IV. Cross resistance of Bacillus megaterium to forty-four antimicrobial drugs. Appl. Microbiol. 2, 57–63 (1954).
Wiegand, I., Hilpert, K. & Hancock, R. E. W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3, 163–175 (2008).
Rhoads, A. & Au, K. F. PacBio sequencing and its applications. Genomics Proteomics Bioinformatics 13, 278–289 (2015).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Oksanen, J. et al. vegan: community ecology package (2017); https://cran.r-project.org/web/packages/vegan/index.html
Chakravorty, S., Helb, D., Burday, M., Connell, N. & Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 69, 330–339 (2007).
Fisher, R. A., Corbet, A. S. & Williams, C. B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 12, 42 (1943).
Simpson, E. H. Measurement of diversity. Nature 163, 688 (1949).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA–Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Jonsson, V., Österlund, T., Nerman, O. & Kristiansson, E. Statistical evaluation of methods for identification of differentially abundant genes in comparative metagenomics. BMC Genomics 17, 78 (2016).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA–seq data. Genome Biol. 11, R25 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Source J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Sambrook, J. & Russell, D. W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2001).
Wang, X., McGrath, S. C., Cotter, R. J. & Raetz, C. R. H. Expression cloning and periplasmic orientation of the Francisella novicida lipid A 4′-phosphatase LpxF. J. Biol. Chem. 281, 9321–9330 (2006).
Rossetti, F. F. et al. Interaction of poly(l-lysine)-g-poly(ethylene glycol) with supported phospholipid bilayers. Biophys. J. 87, 1711–1721 (2004).
Bacic, M. K. & Smith, C. J. Laboratory maintenance and cultication of Bacteroides species. Curr. Protoc. Microbiol. 13, 13C.1 (2008).
Lozupone, C. A., Hamady, M., Kelley, S. T. & Knight, R. Quantitative and qualitative diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73, 1576–1585 (2007).
Acknowledgements
The authors thank D. Módos, D. Fazekas, K. Kovács, A. Tooming-Klunderud, J. Sóki and E. Urbán for technical support. This work was supported by the ‘Lendület’ programme of the Hungarian Academy of Sciences (B.P. and C.P.), the Wellcome Trust (B.P.), The European Research Council H2020-ERC-2014-CoG 648364 Resistance Evolution (C.P.), GINOP 2.3.2-15-2016-00014 (EVOMER, C.P. and B.P.), GINOP-2.3.2-15-2016-00020 (MolMedEx TUMORDNS, C.P.), GINOP-2.3.2-15-2016-00026 (iChamber, B.P.), the National Research, Development and Innovation Office, Hungary (NKFIH grant K120220, B.K.), NKFIH grants FK124254 (O.M.) and KH125616 (B.P.), and a PhD fellowship from the Boehringer Ingelheim Fonds (A.N.). B.K. holds a Bolyai Janos Scholarship. The Pacific Biosciences sequencing service was provided by the Norwegian Sequencing Centre, a national technology platform hosted by the University of Oslo and supported by the ‘Functional Genomics’ and ‘Infrastructure’ programs of the Research Council of Norway and the Southeastern Regional Health Authorities.
Author information
Authors and Affiliations
Contributions
B.K. and C.P. conceived of the project. B.K., O.M., E.A., A.N., B.P. and C.P. planned the experiments and data analyses. O.M., M.S., P.K.J. and R.T. performed the experiments. I.N. performed Illumina sequencing. A.B. and T.M. were responsible for faecal sample collection. B.K., O.M., E.A., A.G., F.P., G.F., I.L., B.B., B.M.V and M.B. analysed the experimental data and carried out bioinformatic analyses. B.K., O.M., B.P. and C.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
B.M.V., B.B. and I.N. had consulting positions at SeqOmics Biotechnology Ltd at the time the study was conceived. SeqOmics Biotechnology Ltd was not directly involved in the design and execution of the experiments or in the writing of the manuscript. This does not alter the authors’ adherence to all the Nature policies on sharing data and materials. The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13, Supplementary Table 5, Supplementary Table 8, Supplementary Table 9 and Supplementary Table 11.
Supplementary Data
Accession numbers of contigs from PacBio sequencing and 16S rRNA sequences.
Supplementary Table 1
A comprehensive catalogue of previously reported AMP resistance genes, compiled based on literature mining, and antibiotic resistance genes obtained from the CARD database.
Supplementary Table 2
Identification of AMP- and antibiotic-resistance genes in the bacterial genome sequences from which the mobile gene pool was derived.
Supplementary Table 3
Characteristics of the transferred AMP- and antibiotic-resistance genes in the mobile gene pool.
Supplementary Table 4
Identification of AMP- and antibiotic-resistance genes associated with naturally occurring plasmids and ICEs in the human microbiota.
Supplementary Table 6
List of resistance contigs identified from the functional metagenomic selections of the uncultured microbiota with 12 AMPs and 11 small-molecule antibiotics (Supplementary Table 5).
Supplementary Table 7
Bacterial abundances in the untreated and AMP- and antibiotic-resistant microbiota at family and order levels, respectively. Abundances at order and family levels are presented on separate Excel sheets.
Supplementary Table 10
List of resistance contigs identified from the functional selection of the cultured microbiota with 5 AMPs and 5 small-molecule antibiotics (Supplementary Table 5).
Supplementary Table 12
Resistance gains in Escherichia coli and Salmonella enterica provided by a representative set of plasmids carrying AMP- and antibiotic-resistance contigs that were isolated in metagenomic screens.
Supplementary Table 13
List of primers used in this study.
Rights and permissions
About this article
Cite this article
Kintses, B., Méhi, O., Ari, E. et al. Phylogenetic barriers to horizontal transfer of antimicrobial peptide resistance genes in the human gut microbiota. Nat Microbiol 4, 447–458 (2019). https://doi.org/10.1038/s41564-018-0313-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0313-5
This article is cited by
-
Metagenomics reveals the temporal dynamics of the rumen resistome and microbiome in goat kids
Microbiome (2024)
-
Efficient in planta production of amidated antimicrobial peptides that are active against drug-resistant ESKAPE pathogens
Nature Communications (2023)
-
Identification of antimicrobial peptides from the human gut microbiome using deep learning
Nature Biotechnology (2022)
-
Examining horizontal gene transfer in microbial communities
Nature Reviews Microbiology (2021)
-
Mobile resistome of human gut and pathogen drives anthropogenic bloom of antibiotic resistance
Microbiome (2020)