Abstract
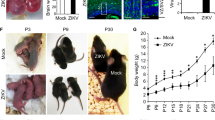
Congenital Zika virus (ZIKV) syndrome may cause fetal microcephaly in ~1% of affected newborns. Here, we investigate whether the majority of clinically inapparent newborns might suffer from long-term health impairments not readily visible at birth. Infection of immunocompetent pregnant mice with high-dose ZIKV caused severe offspring phenotypes, such as fetal death, as expected. By contrast, low-dose (LD) maternal ZIKV infection resulted in reduced fetal birth weight but no other obvious phenotypes. Male offspring born to LD ZIKV-infected mothers had increased testosterone (TST) levels and were less likely to survive in utero infection compared to their female littermates. Males also presented an increased number of immature neurons in apical and basal hippocampal dendrites, while female offspring had immature neurons in basal dendrites only. Moreover, male offspring with high but not very high (storm) TST levels were more likely to suffer from learning and memory impairments compared to females. Future studies are required to understand the impact of TST on neuropathological and neurocognitive impairments in later life. In summary, increased sex-specific vigilance is required in countries with high ZIKV prevalence, where impaired neurodevelopment may be camouflaged by a healthy appearance at birth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Accession numbers of the ZIKV strains used to perform the phylogenetic analysis are indicated in Supplementary Fig. 1. The data that support the findings of this study are available from the corresponding author on request.
References
Dick, G. W., Kitchen, S. F. & Haddow, A. J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 46, 509–520 (1952).
Driggers, R. W. et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N. Engl. J. Med. 374, 2142–2151 (2016).
Faria, N. R. et al. Zika virus in the Americas: early epidemiological and genetic findings. Science 352, 345–349 (2016).
França, G. V. et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet 388, 891–897 (2016).
van der Eijk, A. A. Miscarriage associated with Zika virus infection. N. Engl. J. Med. 375, 1002–1004 (2016).
Brasil, P. et al. Zika virus infection in pregnant women in Rio de Janeiro. N. Engl. J. Med. 375, 2321–2334 (2016).
Cauchemez, S. et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 387, 2125–2132 (2016).
Kapogiannis, B. G., Chakhtoura, N., Hazra, R. & Spong, C. Y. Bridging knowledge gaps to understand how Zika virus exposure and infection affect child development. JAMA Pediatr. 171, 478–485 (2017).
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016).
Miner, J. J. et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165, 1081–1091 (2016).
Li, C. et al. Zika virus disrupts neural progenitor development and leads to microcephaly in mice. Cell Stem Cell 19, 120–126 (2016).
Yockey, L. J. et al. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell 166, 1247–1256 (2016).
Engels, G. et al. Pregnancy-related immune adaptation promotes the emergence of highly virulent H1N1 influenza virus strains in allogenically pregnant mice. Cell Host Microbe 21, 321–333 (2017).
Shin Yim, Y. et al. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature 549, 482–487 (2017).
Estes, M. L. & McAllister, A. K. Maternal immune activation: implications for neuropsychiatric disorders. Science 353, 772–777 (2016).
Leuner, B. & Shors, T. J. New spines, new memories. Mol. Neurobiol. 29, 117–130 (2004).
Maliqueo, M., Echiburú, B. & Crisosto, N. Sex steroids modulate uterine–placental vasculature: implications for obstetrics and neonatal outcomes. Front. Physiol. 7, 152 (2016).
Shors, T. J. & Miesegaes, G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc. Natl Acad. Sci. USA 99, 13955–13960 (2002).
Barbazanges, A., Piazza, P. V., Le Moal, M. & Maccari, S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J. Neurosci. 16, 3943–3949 (1996).
Li, H. et al. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell 19, 593–598 (2016).
Coan, P. M. et al. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. 586, 4567–4576 (2008).
He, S., Allen, J. C. Jr., Malhotra, R., Østbye, T. & Tan, T. C. Association of maternal serum progesterone in early pregnancy with low birth weight and other adverse pregnancy outcomes. J. Matern. Fetal Neonatal Med. 29, 1999–2004 (2016).
Aiken, C. E. & Ozanne, S. E. Sex differences in developmental programming models. Reproduction 145, R1–R13 (2013).
Manikkam, M. et al. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology 145, 790–798 (2004).
Meikle, D. B. & Drickamer, L. C. Food availability and secondary sex ratio variation in wild and laboratory house mice (Mus musculus). J. Reprod. Fertil. 78, 587–591 (1986).
Nguyen, T. V. et al. Sex-specific associations of testosterone with prefrontal-hippocampal development and executive function. Psychoneuroendocrinology 76, 206–217 (2017).
Miyamoto, A. et al. Microglia contact induces synapse formation in developing somatosensory cortex. Nat. Commun. 7, 12540 (2016).
Miner, J. J. et al. Zika virus infection in mice causes panuveitis with shedding of virus in tears. Cell Rep. 16, 3208–3218 (2016).
van den Pol, A. N., Mao, G., Yang, Y., Ornaghi, S. & Davis, J. N. Zika virus targeting in the developing brain. J. Neurosci. 37, 2161–2175 2017).
Anacker, C. & Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—linking memory and mood. Nat. Rev. Neurosci. 18, 335–346 (2017).
D’Hooge, R. & De Deyn, P. P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Rev. 36, 60–90 (2001).
Janus, C. Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn. Mem. 11, 337–346 (2004).
Brody, D. L. & Holtzman, D. M. Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp. Neurol. 197, 330–340 (2006).
Lee, A. S., Duman, R. S. & Pittenger, C. A double dissociation revealing bidirectional competition between striatum and hippocampus during learning. Proc. Natl Acad. Sci. USA 105, 17163–17168 (2008).
Sabuncu, M. R. et al. Morphometricity as a measure of the neuroanatomical signature of a trait. Proc. Natl Acad. Sci. USA 113, E5749–E5756 (2016).
Celec, P., Ostatníková, D. & Hodosy, J. On the effects of testosterone on brain behavioral functions. Front. Neurosci. 9, 12 (2015).
Adams Waldorf, K. M. et al. Congenital Zika virus infection as a silent pathology with loss of neurogenic output in the fetal brain. Nat. Med. 24, 368–374 (2018).
Russell, P. K., Udomsakdi, S. & Halstead, S. B. Antibody response in dengue and dengue hemorrhagic fever. Jpn. J. Med. Sci. Biol. 20, 103–108 (1967).
Styer, L. M. et al. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3, 1262–1270 (2007).
Croy, B. A., Yamada, A. T., DeMayo, F. J. & Adamson, S. L. The Guide to Investigation of Mouse Pregnancy (Academic, San Diego, CA, 2014).
Tappe, D. et al. Ross River virus infection in a traveller returning from northern Australia. Med. Microbiol. Immunol. 198, 271–273 (2009).
Solano, M. E., Thiele, K., Kowal, M. K. & Arck, P. C. Identification of suitable reference genes in the mouse placenta. Placenta 39, 7–15 (2016).
Mu, J., Slevin, J. C., Qu, D., McCormick, S. & Adamson, S. L. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod. Biol. Endocrinol. 6, 34 (2008).
Paxinos, G. & Franklin, K. B. J. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates 4th edn (Elsevier, Amsterdam, 2012).
Risher, W. C., Ustunkaya, T., Singh Alvarado, J. & Eroglu, C. Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS ONE 9, e107591 (2014).
Ferreira, T. A. et al. Neuronal morphometry directly from bitmap images. Nat. Methods 11, 982–984 (2014).
Hölter, S. M. et al. Tests for anxiety-related behavior in mice. Curr. Protoc. Mouse Biol. 5, 291–309 (2015).
Hölter, S. M. et al. Assessing cognition in mice. Curr. Protoc. Mouse Biol. 5, 331–358 (2015).
Mui, A. M. et al. Daily visual stimulation in the critical period enhances multiple aspects of vision through BDNF-mediated pathways in the mouse retina. PLoS ONE 13, e0192435 (2018).
Prusky, G. T., Alam, N. M., Beekman, S. & Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest. Ophthalmol. Vis. Sci. 45, 4611–4616 (2004).
Gröticke, I., Hoffmann, K. & Löscher, W. Behavioral alterations in a mouse model of temporal lobe epilepsy induced by intrahippocampal injection of kainate. Exp. Neurol. 213, 71–83 (2008).
Guindon, S. et al. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321 (2010).
Acknowledgements
The Heinrich Pette Institute, Leibniz Institute for Experimental Virology is supported by the Free and Hanseatic City of Hamburg and the Federal Ministry of Health. This study was supported by the Federal Ministry of Health (G.G.), the German Research Center for Infection (DZIF) (G.G.), the Niedersachsen-Research Network on Neuroinfectiology (N-RENNT) of the Ministry of Science and Culture of Lower Saxony, Germany (W.B., W.L.) and the German Federal Ministry of Education and Research (Infrafrontier grant 01KX1012) (M.H.A.). F.C.d.A. is supported by Deutsche Forschungsgemeinschaft (DFG) Grant (FOR 2419, CA1495/1-1 and CA 1495/4-1), ERA-NET Neuron Grant (Bundesministerium für Bildung und Forschung, BMBF, 01EW1410 ZMNH AN B1), Landesforschungsförderung Hamburg (Z-AN LF) and University Medical Center Hamburg-Eppendorf (UKE). R.B. is supported by the Deutsche Forschungsgemeinschaft (BA 1505/8-1). We are grateful for the excellent technical contribution of all staff at the technology platform small animal models of the Heinrich Pette Institute, Leibniz Institute for Experimental Virology, Hamburg and the technical staff of the Department of Pathology, University of Veterinary Medicine, Hannover. We thank T. Andreas from the Department of Obstetrics and Fetal Medicine at the University Medical Center Hamburg-Eppendorf for his excellent technical support with the preparation of pregnant mice. We thank P. Pruunsild and H. Bading, Department of Neurobiology, Interdisciplinary Center for Neurosciences at Heidelberg University for providing human NPCs. We thank U. Markert, Department of Obstetrics, Placenta-Lab, University Hospital Jena for providing the human placental cells.
Author information
Authors and Affiliations
Contributions
G.G. conceived the study. S.S.-B. and G.G. designed and coordinated the experiments. S.S.-B., K.W.-G., C.D., A.P., G.P.-S., U.M. and S.Thi. performed all infection studies in mice. TST treatment of mice was performed by H.L., J.S. and S.H. S.Tha., I.A.A., T.S., N.M.K., C.D. and K.W.-G. performed the qRT–PCR assays. S.Tha., C.D., K.W.-G. and A.P. performed the hormone ELISA as well as the cytokine assays. T.R. measured the TST levels. V.H., W.B., V.M.P. and I.G. performed the histological and immunohistological examinations, TUNEL and cytokeratin staining, and the in situ hybridization and analysis. B.S. performed the Iba1 staining and respective analysis of fetal brains. H.I. performed the MRI scans on the brains of the offspring. M.R. performed Golgi staining and assisted with spine analysis. R.S. performed image acquisition, and the dendritic and spine analyses. F.C.d.A. coordinated the brain analysis. U.B. performed the immunohistochemical analyses of retinas. T.M. and R.B. performed the ZIKV replication kinetics in cell culture. Histopathological findings were analysed and discussed by P.A., M.A.F., V.H., I.G., V.M.P. and W.B. S.J. and T.S. measured viral IgG and IgM titres. D.C. performed the phylogenetic analysis. S.M.H., O.A., F.M., V.K., R.D., L.S., W.L., I.W. and C.K. performed and/or analysed the behavioural experiments. H.F. and M.H.d.A. coordinated and conceived the animal phenotypic tests. S.B. and L.R. were responsible for the recruitment of the mother–child cohort in Iquitos, Peru and for the qRT–PCR analysis of patient sera. J.S.-C., O.V. and M.G. provided material, analysed the data and discussed the study. S.S.-B. and G.G. wrote the manuscript. All authors revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–3, Supplementary Figures 1–13
Rights and permissions
About this article
Cite this article
Stanelle-Bertram, S., Walendy-Gnirß, K., Speiseder, T. et al. Male offspring born to mildly ZIKV-infected mice are at risk of developing neurocognitive disorders in adulthood. Nat Microbiol 3, 1161–1174 (2018). https://doi.org/10.1038/s41564-018-0236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0236-1
This article is cited by
-
Zika virus-induced TNF-α signaling dysregulates expression of neurologic genes associated with psychiatric disorders
Journal of Neuroinflammation (2022)
-
Neurocognitive impacts of arbovirus infections
Journal of Neuroinflammation (2020)