Abstract
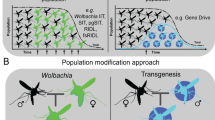
Human pathogens that are transmitted by insects are a global problem, particularly those vectored by mosquitoes; for example, malaria parasites transmitted by Anopheles species, and viruses such as dengue, Zika and chikungunya that are carried by Aedes mosquitoes. Over the past 15 years, the prevalence of malaria has been substantially reduced and virus outbreaks have been contained by controlling mosquito vectors using insecticide-based approaches. However, disease control is now threatened by alarming rates of insecticide resistance in insect populations, prompting the need to develop a new generation of specific strategies that can reduce vector-mediated transmission. Here, we review how increased knowledge in insect biology and insect–pathogen interactions is stimulating new concepts and tools for vector control. We focus on strategies that either interfere with the development of pathogens within their vectors or directly impact insect survival, including enhancement of vector-mediated immune control, manipulation of the insect microbiome, or use of powerful new genetic tools such as CRISPR–Cas systems to edit vector genomes. Finally, we offer a perspective on the implementation hurdles as well as the knowledge gaps that must be filled in the coming years to safely realize the potential of these novel strategies to eliminate the scourge of vector-borne disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kwiatkowski, D. P. How malaria has affected the human genome and what human genetics can teach us about malaria. Am. J. Hum. Genet. 77, 171–192 (2005).
Kwiatkowski, D. Malaria genomics: tracking a diverse and evolving parasite population. Int. Health 7, 82–84 (2015).
World Malaria Report 196 (WHO, Geneva, 2017).
The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 8, e1000401 (2011).
Neafsey, D. E. et al. Mosquito genomics. Highly evolvable malaria vectors: the genomes of 16 Anopheles mosquitoes. Science 347, 1258522 (2015).
Zeldenryk, L. M., Gray, M., Speare, R., Gordon, S. & Melrose, W. The emerging story of disability associated with lymphatic filariasis: a critical review. PLoS Negl. Trop. Dis. 5, e1366 (2011).
Bern, C., Maguire, J. H. & Alvar, J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis. 2, e313 (2008).
Garza, M. et al. Projected future distributions of vectors of Trypanosoma cruzi in North America under climate change scenarios. PLoS Negl. Trop. Dis. 8, e2818 (2014).
Gascon, J., Bern, C. & Pinazo, M. J. Chagas disease in Spain, the United States and other non-endemic countries. Acta Trop. 115, 22–27 (2010).
Ioos, S. et al. Current Zika virus epidemiology and recent epidemics. Med. Mal. Infect. 44, 302–307 (2014).
Faria, N. R. et al. Zika virus in the Americas: early epidemiological and genetic findings. Science 352, 345–349 (2016).
Reiter, P. & Sprenger, D. The used tire trade: a mechanism for the worldwide dispersal of container breeding mosquitoes. J. Am. Mosq. Control Assoc. 3, 494–501 (1987).
Rezza, G. et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370, 1840–1846 (2007).
Lednicky, J. et al. Mayaro virus in child with acute febrile illness, Haiti, 2015. Emerg. Infect. Dis. 22, 2000–2002 (2016).
Hotez, P. J. & Murray, K. O. Dengue, West Nile virus, chikungunya, Zika—and now Mayaro? PLoS Negl. Trop. Dis. 11, e0005462 (2017).
Bhatt, S. et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 526, 207–211 (2015).
WHO Malaria fact sheet #94, December 2011 (Innovative Vector Control Consortium); www.ivcc.com/who-malaria-fact-sheet
Franco, J. R. et al. Monitoring the elimination of human African trypanosomiasis: update to 2014. PLoS Negl. Trop. Dis. 11, e0005585 (2017).
Investing to Overcome the Global Impact of Neglected Tropical Diseases—Third WHO Report on Neglected Tropical Diseases 191 (WHO, Geneva, 2015).
Ranson, H. & Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 32, 187–196 (2016).
Moyes, C. L. et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 11, e0005625 (2017).
Gomes, B. et al. Knockdown resistance mutations predict DDT resistance and pyrethroid tolerance in the visceral leishmaniasis vector Phlebotomus argentipes. PLoS Negl. Trop. Dis. 11, e0005504 (2017).
Morrison, A. C., Zielinski-Gutierrez, E., Scott, T. W. & Rosenberg, R. Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med. 5, e68 (2008).
Killeen, G. F. Characterizing, controlling and eliminating residual malaria transmission. Malar. J. 13, 330 (2014).
Nene, V. et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316, 1718–1723 (2007).
Dudchenko, O. et al. De novo assembly of the Aedes aegypti genome using Hi-C yields chromosome-length scaffolds. Science 356, 92–95 (2017).
Chen, X. G. et al. Genome sequence of the Asian Tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc. Natl Acad. Sci. USA 112, E5907–5915 (2015).
Smidler, A. L., Terenzi, O., Soichot, J., Levashina, E. A. & Marois, E. Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS ONE 8, e74511 (2013).
Dong, S. et al. Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito, Aedes aegypti. PLoS ONE 10, e0122353 (2015).
Itokawa, K., Komagata, O., Kasai, S., Ogawa, K. & Tomita, T. Testing the causality between CYP9M10 and pyrethroid resistance using the TALEN and CRISPR/Cas9 technologies. Sci. Rep. 6, 24652 (2016).
Gantz, V. M. et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl Acad. Sci. USA 112, E6736–6743 (2015).
Hammond, A. et al. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol. 34, 78–83 (2016).
Li, M. et al. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector, Aedes aegypti. Proc. Natl Acad. Sci. USA 114, E10540–E10549 (2017).
Dodson, B. L. & Rasgon, J. L. Vector competence of Anopheles and Culex mosquitoes for Zika virus. PeerJ 5, e3096 (2017).
Vanlandingham, D. L. et al. Differential infectivities of o’nyong-nyong and chikungunya virus isolates in Anopheles gambiae and Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 72, 616–621 (2005).
Macdonald, G. Epidemiological basis of malaria control. Bull. World Health Organ. 15, 613–626 (1956).
Jordan, A. M. & Curtis, C. F. Productivity of Glossina morsitans morsitans Westwood maintained in the laboratory, with particular reference to the sterile-insect release method. Bull. World Health Organ. 46, 33–38 (1972).
Tobe, S. S. & Langley, P. A. Reproductive physiology of Glossina. Ann. Rev. Entomol. 23, 283–307 (1978).
Knols, B. G., Willemse, L., Flint, S. & Mate, A. A trial to control the tsetse fly, Glossina morsitans centralis, with low densities of odour-baited targets in west Zambia. Med. Vet. Entomol. 7, 161–169 (1993).
Gurtler, R. E. et al. Domestic animal hosts strongly influence human-feeding rates of the Chagas disease vector Triatoma infestans in Argentina. PLoS Negl. Trop. Dis. 8, e2894 (2014).
Beach, R. Mosquitoes: biting behavior inhibited by ecdysone. Science 205, 829–831 (1979).
Takken, W., Klowden, M. J. & Chambers, G. M. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J. Med. Entomol. 35, 639–645 (1998).
Lefèvre, T., Vantaux, A., Dabiré, K. R., Mouline, K. & Cohuet, A. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 9, e1003365 (2013).
Cirimotich, C. M. et al. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858 (2011).
Takken, W. et al. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites Vectors 6, 345 (2013).
Blanford, S. et al. Fungal pathogen reduces potential for malaria transmission. Science 308, 1638–1641 (2005).
Chan, M. & Johansson, M. A. The incubation periods of Dengue viruses. PLoS ONE 7, e50972 (2012).
Ye, Y. H. et al. Wolbachia reduces the transmission potential of Dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis. 9, e0003894 (2015).
Lindsay, S. W. et al. Ability of Anopheles gambiae mosquitoes to transmit malaria during the dry and wet seasons in an area of irrigated rice cultivation in The Gambia. J. Trop. Med. Hyg. 94, 313–324 (1991).
Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition (WHO, 2009).
Achee, N. L. et al. A critical assessment of vector control for dengue prevention. PLoS Negl. Trop. Dis. 9, e0003655 (2015).
Martinez-Torres, D. et al. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 7, 179–184 (1998).
Martinez-Torres, D. et al. Voltage-dependent Na+ channels in pyrethroid-resistant Culex pipiens L mosquitoes. Pestic. Sci. 55, 1012–1020 (1999).
Ranson, H. et al. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 9, 491–497 (2000).
Norris, L. C. et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc. Natl Acad. Sci. USA 112, 815–820 (2015).
Toe, K. H. et al. Increased pyrethroid resistance in malaria vectors and decreased bed net effectiveness, Burkina Faso. Emerg. Infect. Dis. 20, 1691–1696 (2014).
Poupardin, R., Srisukontarat, W., Yunta, C. & Ranson, H. Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti. PLoS Negl. Trop. Dis. 8, e2743 (2014).
Bariami, V., Jones, C. M., Poupardin, R., Vontas, J. & Ranson, H. Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1692 (2012).
Grigoraki, L. et al. Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian Tiger mosquito Aedes albopictus. PLoS Negl. Trop. Dis. 9, e0003771 (2015).
Balabanidou, V. et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc. Natl Acad. Sci. USA 113, 9268–9273 (2016).
Viana, M., Hughes, A., Matthiopoulos, J., Ranson, H. & Ferguson, H. M. Delayed mortality effects cut the malaria transmission potential of insecticide-resistant mosquitoes. Proc. Natl Acad. Sci. USA 113, 8975–8980 (2016).
Martins, A. J. et al. Effect of insecticide resistance on development, longevity and reproduction of field or laboratory selected Aedes aegypti populations. PLoS ONE 7, e31889 (2012).
Baldini, F. et al. The interaction between a sexually transferred steroid hormone and a female protein regulates oogenesis in the malaria mosquito Anopheles gambiae. PLoS Biol. 11, e1001695 (2013).
Gabrieli, P. et al. Sexual transfer of the steroid hormone 20E induces the postmating switch in Anopheles gambiae. Proc. Natl Acad. Sci. USA 111, 16353–16358 (2014).
Mitchell, S. N. et al. Mosquito biology. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347, 985–988 (2015).
Pitts, R. J., Liu, C., Zhou, X., Malpartida, J. C. & Zwiebel, L. J. Odorant receptor-mediated sperm activation in disease vector mosquitoes. Proc. Natl Acad. Sci. USA 111, 2566–2571 (2014).
Clifton, M. E., Correa, S., Rivera-Perez, C., Nouzova, M. & Noriega, F. G. Male Aedes aegypti mosquitoes use JH III transferred during copulation to influence previtellogenic ovary physiology and affect the reproductive output of female mosquitoes. J. Insect Physiol. 64, 40–47 (2014).
Harris, C. et al. Sterilising effects of pyriproxyfen on Anopheles arabiensis and its potential use in malaria control. Parasit. Vectors 6, 144 (2013).
Lwetoijera, D. W. et al. Comprehensive sterilization of malaria vectors using pyriproxyfen: a step closer to malaria elimination. Am. J. Trop. Med. Hyg. 90, 852–855 (2014).
Kawada, H. et al. A small-scale field trial of pyriproxyfen-impregnated bed nets against pyrethroid-resistant Anopheles gambiae s.s. in western Kenya. PLoS ONE 9, e111195 (2014).
Ohashi, K. et al. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae). J. Med. Entomol. 49, 1052–1058 (2012).
Ngufor, C. et al. Olyset Duo(R) (a pyriproxyfen and permethrin mixture net): an experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in Southern Benin. PLoS ONE 9, e93603 (2014).
Childs, L. M. et al. Disrupting mosquito reproduction and parasite development for malaria control. PLoS Pathog. 12, e1006060 (2016).
Tiono, A. B. et al. The AvecNet Trial to assess whether addition of pyriproxyfen, an insect juvenile hormone mimic, to long-lasting insecticidal mosquito nets provides additional protection against clinical malaria over current best practice in an area with pyrethroid-resistant vectors in rural Burkina Faso: study protocol for a randomised controlled trial. Trials 16, 113 (2015).
Shin, S. W. et al. REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J. Biol. Chem. 280, 16499–16507 (2005).
Bian, G., Shin, S. W., Cheon, H. M., Kokoza, V. & Raikhel, A. S. Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA 102, 13568–13573 (2005).
Frolet, C., Thoma, M., Blandin, S., Hoffmann, J. A. & Levashina, E. A. Boosting NF-κB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25, 677–685 (2006).
Garver, L. S., Dong, Y. & Dimopoulos, G. Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog. 5, e1000335 (2009).
Meister, S. et al. Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites. PLoS Pathog. 5, e1000542 (2009).
Mitri, C. et al. Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog. 5, e1000576 (2009).
Dong, Y. et al. Engineered anopheles immunity to Plasmodium infection. PLoS Pathog. 7, e1002458 (2011).
Zou, Z. et al. Transcriptome analysis of Aedes aegypti transgenic mosquitoes with altered immunity. PLoS Pathog. 7, e1002394 (2011).
Antonova, Y., Alvarez, K. S., Kim, Y. J., Kokoza, V. & Raikhel, A. S. The role of NF-κB factor REL2 in the Aedes aegypti immune response. Insect Biochem. Mol. Biol. 39, 303–314 (2009).
Castillo, J. C., Ferreira, A. B. B., Trisnadi, N. & Barillas-Mury, C. Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci. Immunol. 2, eaal1505 (2017).
Smith, R. C. & Barillas-Mury, C. Plasmodium oocysts: overlooked targets of mosquito immunity. Trends Parasitol. 32, 979–990 (2016).
Gupta, L. et al. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5, 498–507 (2009).
Smith, R. C., Barillas-Mury, C. & Jacobs-Lorena, M. Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc. Natl Acad. Sci. USA 112, E3412–3420 (2015).
Olson, K. E. & Blair, C. D. Arbovirus-mosquito interactions: RNAi pathway. Curr. Opin. Virol. 15, 119–126 (2015).
Morazzani, E. M., Wiley, M. R., Murreddu, M. G., Adelman, Z. N. & Myles, K. M. Production of virus-derived ping-pong-dependent piRNA-like small RNAs in the mosquito soma. PLoS Pathog. 8, e1002470 (2012).
Vodovar, N. et al. Arbovirus-derived piRNAs exhibit a ping-pong signature in mosquito cells. PLoS ONE 7, e30861 (2012).
Hess, A. M. et al. Small RNA profiling of Dengue virus-mosquito interactions implicates the PIWI RNA pathway in anti-viral defense. BMC Microbiol. 11, 45 (2011).
Miesen, P., Girardi, E. & van Rij, R. P. Distinct sets of PIWI proteins produce arbovirus and transposon-derived piRNAs in Aedes aegypti mosquito cells. Nucleic Acids Res. 43, 6545–6556 (2015).
Xi, Z., Ramirez, J. L. & Dimopoulos, G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 4, e1000098 (2008).
Souza-Neto, J. A., Sim, S. & Dimopoulos, G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl Acad. Sci. USA 106, 17841–17846 (2009).
Ramirez, J. L. & Dimopoulos, G. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev. Comp. Immunol. 34, 625–629 (2010).
Sim, S. et al. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl. Trop. Dis. 7, e2295 (2013).
Palmer, W. H., Varghese, F. S. & van Rij, R. P. Natural variation in resistance to virus infection in Dipteran insects. Viruses 10, 118 (2018).
Kokoza, V. et al. Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc. Natl Acad. Sci. USA 97, 9144–9149 (2000).
Kim, W. et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium. J. Med. Entomol. 41, 447–455 (2004).
Jupatanakul, N. et al. Engineered Aedes aegypti JAK/STAT pathway-mediated immunity to Dengue virus. PLoS Negl. Trop. Dis. 11, e0005187 (2017).
Isaacs, A. T. et al. Transgenic Anopheles stephensi coexpressing single-chain antibodies resist Plasmodium falciparum development. Proc. Natl Acad. Sci. USA 109, E1922–1930 (2012).
Clayton, A. M., Cirimotich, C. M., Dong, Y. & Dimopoulos, G. Caudal is a negative regulator of the Anopheles IMD pathway that controls resistance to Plasmodium falciparum infection. Dev. Comp. Immunol. 39, 323–332 (2013).
Garver, L. S., de Almeida Oliveira, G. & Barillas-Mury, C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 9, e1003622 (2013).
Dong, Y., Cirimotich, C. M., Pike, A., Chandra, R. & Dimopoulos, G. Anopheles NF-κB-regulated splicing factors direct pathogen-specific repertoires of the hypervariable pattern recognition receptor AgDscam. Cell Host Microbe 12, 521–530 (2012).
Smith, R. C., Kizito, C., Rasgon, J. L. & Jacobs-Lorena, M. Transgenic mosquitoes expressing a phospholipase A(2) gene have a fitness advantage when fed Plasmodium falciparum-infected blood. PLoS ONE 8, e76097 (2013).
Franz, A. W. et al. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti. Proc. Natl Acad. Sci. USA 103, 4198–4203 (2006).
Pike, A. et al. Changes in the microbiota cause genetically modified Anopheles to spread in a population. Science 357, 1396–1399 (2017).
Ito, J., Ghosh, A., Moreira, L. A., Wimmer, E. A. & Jacobs-Lorena, M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature 417, 452–455 (2002).
Ghosh, A. K., Coppens, I., Gardsvoll, H., Ploug, M. & Jacobs-Lorena, M. Plasmodium ookinetes coopt mammalian plasminogen to invade the mosquito midgut. Proc. Natl Acad. Sci. USA 108, 17153–17158 (2011).
Vega-Rodriguez, J. et al. Multiple pathways for Plasmodium ookinete invasion of the mosquito midgut. Proc. Natl Acad. Sci. USA 111, E492–500 (2014).
Ghosh, A. K. et al. Malaria parasite invasion of the mosquito salivary gland requires interaction between the Plasmodium TRAP and the Anopheles saglin proteins. PLoS Pathog. 5, e1000265 (2009).
Clayton, A. M., Dong, Y. & Dimopoulos, G. The Anopheles innate immune system in the defense against malaria infection. J. Innate Immun. 6, 169–181 (2014).
Molina-Cruz, A. et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984–987 (2013).
Smith, R. C. & Jacobs-Lorena, M. Malaria parasite Pfs47 disrupts JNK signaling to escape mosquito immunity. Proc. Natl Acad. Sci. USA 112, 1250–1251 (2015).
Molina-Cruz, A. et al. Plasmodium evasion of mosquito immunity and global malaria transmission: the lock-and-key theory. Proc. Natl Acad. Sci. USA 112, 15178–15183 (2015).
Dillon, R. J. & Dillon, V. M. The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 (2004).
Dale, C. & Moran, N. A. Molecular interactions between bacterial symbionts and their hosts. Cell 126, 453–465 (2006).
Douglas, A. E. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37 (1998).
Dong, Y., Manfredini, F. & Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5, e1000423 (2009).
Wang, Y., Gilbreath, T. M. 3rd, Kukutla, P., Yan, G. & Xu, J. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS ONE 6, e24767 (2011).
Boissière, A. et al. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 8, e1002742 (2012).
da Mota, F. F. et al. Cultivation-independent methods reveal differences among bacterial gut microbiota in triatomine vectors of Chagas disease. PLoS Negl. Trop. Dis. 6, e1631 (2012).
Aksoy, E. et al. Analysis of multiple tsetse fly populations in Uganda reveals limited diversity and species-specific gut microbiota. Appl. Environ. Microbiol. 80, 4301–4312 (2014).
Segata, N. et al. The reproductive tracts of two malaria vectors are populated by a core microbiome and by gender- and swarm-enriched microbial biomarkers. Sci. Rep. 6, 24207 (2016).
Fraihi, W. et al. An integrated overview of the midgut bacterial flora composition of Phlebotomus perniciosus, a vector of zoonotic visceral leishmaniasis in the Western Mediterranean Basin. PLoS Negl. Trop. Dis. 11, e0005484 (2017).
Azambuja, P., Feder, D. & Garcia, E. S. Isolation of Serratia marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 107, 89–96 (2004).
Moraes, C. S. et al. Prodigiosin is not a determinant factor in lysis of Leishmania (Viannia) braziliensis after interaction with Serratia marcescens D-mannose sensitive fimbriae. Exp. Parasitol. 122, 84–90 (2009).
Bando, H. et al. Intra-specific diversity of Serratia marcescens in Anopheles mosquito midgut defines Plasmodium transmission capacity. Sci. Rep. 3, 1641 (2013).
Bahia, A. C. et al. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ. Microbiol. 16, 2980–2994 (2014).
Apte-Deshpande, A., Paingankar, M., Gokhale, M. D. & Deobagkar, D. N. Serratia odorifera a midgut inhabitant of Aedes aegypti mosquito enhances its susceptibility to dengue-2 virus. PLoS ONE 7, e40401 (2012).
Carissimo, G. et al. Antiviral immunity of Anopheles gambiae is highly compartmentalized, with distinct roles for RNA interference and gut microbiota. Proc. Natl Acad. Sci. USA 112, 176–185 (2015).
Scholte, E. J. et al. An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308, 1641–1642 (2005).
Fieck, A., Hurwitz, I., Kang, A. S. & Durvasula, R. Trypanosoma cruzi: synergistic cytotoxicity of multiple amphipathic anti-microbial peptides to T. cruzi and potential bacterial hosts. Exp. Parasitol. 125, 342–347 (2010).
Wang, S. et al. Driving mosquito refractoriness to Plasmodium falciparum with engineered symbiotic bacteria. Science 357, 1399–1402 (2017).
Xi, Z., Khoo, C. C. & Dobson, S. L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310, 326–328 (2005).
Bian, G. et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340, 748–751 (2013).
Attardo, G. M. et al. Analysis of milk gland structure and function in Glossina morsitans: milk protein production, symbiont populations and fecundity. J. Insect Physiol. 54, 1236–1242 (2008).
Favia, G. et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl Acad. Sci. USA 104, 9047–9051 (2007).
Hilgenboecker, K., Hammerstein, P., Schlattmann, P., Telschow, A. & Werren, J. H. How many species are infected with Wolbachia?A statistical analysis of current data. FEMS Microbiol. Lett. 281, 215–220 (2008).
Werren, J. H., Baldo, L. & Clark, M. E. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Micro. 6, 741–751 (2008).
LePage, D. P. et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247 (2017).
Beckmann, J. F., Ronau, J. A. & Hochstrasser, M. A Wolbachia deubiquitylating enzyme induces cytoplasmic incompatibility. Nat. Microbiol. 2, 17007 (2017).
Lindsey, A. R. I. et al. Evolutionary genetics of cytoplasmic incompatibility genes cifA and cifB in prophage WO of Wolbachia. Genome Biol. Evol. 10, 434–451 (2018).
Hughes, G. L., Koga, R., Xue, P., Fukatsu, T. & Rasgon, J. L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7, e1002043 (2011).
Kambris, Z. et al. Wolbachia stimulates immune gene expression and inhibits plasmodium development in Anopheles gambiae. PLoS Pathog. 6, e1001143 (2010).
Moreira, L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009).
Glaser, R. L. & Meola, M. A. The native Wolbachia endosymbionts of Drosophila melanogaster and Culex quinquefasciatus increase host resistance to West Nile virus infection. PLoS ONE 5, e11977 (2010).
van den Hurk, A. F. et al. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1892 (2012).
Blagrove, M. S. C., Arias-Goeta, C., Failloux, A. B. & Sinkins, S. P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl Acad. Sci. USA 109, 255–260 (2012).
Dutra, H. L. et al. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774 (2016).
Aliota, M. T., Peinado, S. A., Velez, I. D. & Osorio, J. E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 6, 28792 (2016).
Caragata, E. P. et al. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 9, e1003459 (2013).
Geoghegan, V. et al. Perturbed cholesterol and vesicular trafficking associated with dengue blocking in Wolbachia-infected Aedes aegypti cells. Nat. Commun. 8, 526 (2017).
Brelsfoard, C. L., Sechan, Y. & Dobson, S. L. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2, e129 (2008).
Atyame, C. M. et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl. Trop. Dis. 5, e1440 (2011).
World Mosquito Program (2017); www.worldmosquitoprogram.org
Frentiu, F. D. et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl. Trop. Dis. 8, e2688 (2014).
Schmidt, T. L. et al. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol. 15, e2001894 (2017).
Baldini, F. et al. Evidence of natural Wolbachia infections in field populations of Anopheles gambiae. Nat. Commun. 5, 3985 (2014).
Shaw, W. R. et al. Wolbachia infections in natural Anopheles populations affect egg laying and negatively correlate with Plasmodium development. Nat. Commun. 7, 11772 (2016).
Gomes, F. M. et al. Effect of naturally occurring Wolbachia in Anopheles gambiae s.l. mosquitoes from Mali on Plasmodium falciparum malaria transmission. Proc. Natl Acad. Sci. USA 114, 12566–12571 (2017).
Dodson, B. L. et al. Wolbachia enhances West Nile virus (WNV) infection in the mosquito Culex tarsalis. PLoS Negl. Trop. Dis. 8, e2965 (2014).
Hughes, G. L., Rivero, A. & Rasgon, J. L. Wolbachia can enhance Plasmodium infection in mosquitoes: implications for malaria control? PLoS Pathog. 10, e1004182 (2014).
Burt, A. Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci. 270, 921–928 (2003).
Lindquist, D. A., Abusowa, M. & Hall, M. J. The New World screwworm fly in Libya: a review of its introduction and eradication. Med. Vet. Entomol. 6, 2–8 (1992).
Oliva, C. F. et al. The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS ONE 7, e49414 (2012).
Bellini, R., Medici, A., Puggioli, A., Balestrino, F. & Carrieri, M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J. Med. Entomol. 50, 317–325 (2013).
Vreysen, M. J. et al. Glossina austeni (Diptera: Glossinidae) eradicated on the island of Unguja, Zanzibar, using the sterile insect technique. J. Econ. Entomol. 93, 123–135 (2000).
Dame, D. A., Curtis, C. F., Benedict, M. Q., Robinson, A. S. & Knols, B. G. Historical applications of induced sterilisation in field populations of mosquitoes. Malar. J. 2, S2 (2009).
Harris, A. F. et al. Field performance of engineered male mosquitoes. Nat. Biotechnol. 29, 1034–1037 (2011).
Harris, A. F. et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30, 828–830 (2012).
Carvalho, D. O. et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. Dis. 9, e0003864 (2015).
Chen, C. H. et al. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science 316, 597–600 (2007).
Windbichler, N. et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (2011).
Simoni, A. et al. Development of synthetic selfish elements based on modular nucleases in Drosophila melanogaster. Nucleic Acids Res. 42, 7461–7472 (2014).
Esvelt, K. M., Smidler, A. L., Catteruccia, F. & Church, G. M. Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3, e03401 (2014).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
Hammond, A. M. et al. The creation and selection of mutations resistant to a gene drive over multiple generations in the malaria mosquito. PLoS Genet. 13, e1007039 (2017).
Champer, J. et al. Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLoS Genet. 13, e1006796 (2017).
The Anopheles gambiae 1000 Genomes Consortium. Genetic diversity of the African malaria vector Anopheles gambiae. Nature 552, 96–100 (2017).
Marshall, J. M., Buchman, A., Sanchez, C. H. & Akbari, O. S. Overcoming evolved resistance to population-suppressing homing-based gene drives. Sci. Rep. 7, 3776 (2017).
Sawadogo, S. P. et al. Effects of age and size on Anopheles gambiae s.s. male mosquito mating success. J. Med. Entomol. 50, 285–293 (2013).
Sawadogo, S. P. et al. Targeting male mosquito swarms to control malaria vector density. PLoS ONE 12, e0173273 (2017).
Diabate, A. & Tripet, F. Targeting male mosquito mating behaviour for malaria control. Parasit. Vectors 8, 347 (2015).
Cator, L. J., Arthur, B. J., Harrington, L. C. & Hoy, R. R. Harmonic convergence in the love songs of the dengue vector mosquito. Science 323, 1077–1079 (2009).
Cator, L. J. & Harrington, L. C. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim. Behav. 82, 627–633 (2011).
Oye, K. A. et al. Biotechnology. Regulating gene drives. Science 345, 626–628 (2014).
Ferguson, H. M. et al. Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 7, e1000303 (2010).
Norris, E. J. & Coats, J. R. Current and future repellent technologies: the potential of spatial repellents and their place in mosquito-borne disease control. Int. J. Environ. Res. Public Health 14, 124 (2017).
Ernst, K. C. et al. Awareness and support of release of genetically modified “sterile” mosquitoes, Key West, Florida, USA. Emerg. Infect. Dis. 21, 320–324 (2015).
Adalja, A., Sell, T. K., McGinty, M. & Boddie, C. Genetically modified (gm) mosquito use to reduce mosquito-transmitted disease in the US: a community opinion survey. PLoS Curr. https://doi.org/10.1371/currents.outbreaks.1c39ec05a743d41ee39391ed0f2ed8d3 (2016).
Mathers, C. D., Ezzati, M. & Lopez, A. D. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 1, e114 (2007).
Global Program to Eliminate Lymphatic Filariasis Progress Report 594–608 (WHO, Geneva, 2017).
Lymphatic filariasis (WHO, Geneva, 2017); www.who.int/mediacentre/factsheets/fs102/en/
GBD 2015 Disease and Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1545–1602 (2016).
GBD 2015 Mortality and Cause of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544 (2016).
What is Chagas disease? (WHO, Geneva, 2017); www.who.int/chagas/disease/en/
Control of the leishmaniases — Report of a WHO Expert Committee 158 (World Health Organization, Geneva, 1990).
Parasites—Onchocerciasis (also known as River Blindness) (Centers for Disease Control and Prevention, 2017); www.cdc.gov/parasites/onchocerciasis/epi.html
Brady, O. J. et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 6, e1760 (2012).
Shearer, F. M. et al. Global yellow fever vaccination coverage from 1970 to 2016: an adjusted retrospective analysis. Lancet Infect. Dis. 17, 1209–1217 (2017).
CDC: Zika Travel Information (2017); wwwnc.cdc.gov/travel/page/zika-information
Messina, J. P. et al. Mapping global environmental suitability for Zika virus. eLife 5, e15272 (2016).
Nsoesie, E. O. et al. Global distribution and environmental suitability for chikungunya virus, 1952 to 2015. Euro. Surveill. https://doi.org/10.2807/1560-7917.ES.2016.21.20.30234 (2016).
Chikungunya (WHO, Geneva, 2017); www.who.int/mediacentre/factsheets/fs327/en/
Gibson, G. & Torr, S. J. Visual and olfactory responses of haematophagous Diptera to host stimuli. Med. Vet. Entomol. 13, 2–23 (1999).
Acknowledgements
The authors would like to thank M. Bernardi for graphic assistance and D. Abernathy, P. Marcenac and D. Paton for careful reading of this manuscript. F.C. is funded on research related to the topic discussed here by a Faculty Research Scholar Award by the Howard Hughes Medical Institute (HHMI) and the Bill & Melinda Gates Foundation (BMGF) (grant ID OPP1158190), and by the National Institutes of Health (NIH) (R01 AI124165). The findings and conclusions within this publication are those of the authors and do not necessarily reflect positions or policies of the HHMI, the BMGF or the NIH.
Author information
Authors and Affiliations
Contributions
W.R.S. and F.C. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
Harvard University has filed a patent application on behalf of the investigators related to this research.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shaw, W.R., Catteruccia, F. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat Microbiol 4, 20–34 (2019). https://doi.org/10.1038/s41564-018-0214-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0214-7
This article is cited by
-
mosGILT controls innate immunity and germ cell development in Anopheles gambiae
BMC Genomics (2024)
-
Interkingdom interactions shape the fungal microbiome of mosquitoes
Animal Microbiome (2024)
-
CRISPR-mediated germline mutagenesis for genetic sterilization of Anopheles gambiae males
Scientific Reports (2024)
-
Mosquito larvae exposed to a sublethal dose of photosensitive insecticides have altered juvenile development but unaffected adult life history traits
Parasites & Vectors (2023)
-
Evaluation of the evolutionary genetics and population structure of Culex pipiens pallens in Shandong province, China based on knockdown resistance (kdr) mutations and the mtDNA-COI gene
BMC Genomics (2023)