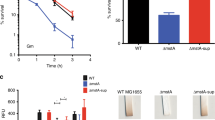
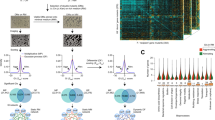
Abstract
Bacteria adapt to environmental stress by producing proteins that provide stress protection. However, stress can severely perturb the kinetics of gene expression, disrupting protein production. Here, we characterized how Escherichia coli mitigates such perturbations under nutrient stress through the kinetic coordination of transcription and translation. We observed that, when translation became limiting under nitrogen starvation, transcription elongation slowed accordingly. This slowdown was mediated by (p)ppGpp, the alarmone whose primary role is thought to be promoter regulation. This kinetic coordination by (p)ppGpp was critical for the robust synthesis of gene products. Surprisingly, under carbon starvation, (p)ppGpp was dispensable for robust synthesis. Characterization of the underlying kinetics revealed that under carbon starvation, transcription became limiting, and translation aided transcription elongation. This mechanism naturally coordinated transcription with translation, alleviating the need for (p)ppGpp as a mediator. These contrasting mechanisms for coordination resulted in the condition-dependent effects of (p)ppGpp on global protein synthesis and starvation survival. Our findings reveal a kinetic aspect of gene expression plasticity, establishing (p)ppGpp as a condition-dependent global effector of gene expression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Browning, D. F. & Busby, S. J. W. Local and global regulation of transcription initiation in bacteria. Nat. Rev. Micro 14, 638–650 (2016).
Schellhorn, H. E. & Hassan, H. M. Transcriptional regulation of katE in Escherichia coli K-12. J. Bacteriol 170, 4286–4292 (1988).
Santos, J. M., Lobo, M., Matos, A. P. A., De Pedro, M. A. & Arraiano, C. M. The gene bolA regulates dacA (PBP5), dacC (PBP6) and ampC (AmpC), promoting normal morphology in Escherichia coli. Mol. Microbiol 45, 1729–1740 (2002).
Mengin-Lecreulx, D. & van Heijenoort, J. Effect of growth conditions on peptidoglycan content and cytoplasmic steps of its biosynthesis in Escherichia coli. J. Bacteriol. 163, 208–212 (1985).
Matin, A., Auger, E. A., Blum, P. H., a. & Schultz, J. E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu. Rev. Microbiol. 43, 293–314 (1989).
Mellies, J., Wise, A. & Villarejo, M. Two different Escherichia coli proP promoters respond to osmotic and growth phase signals. J. Bacteriol. 177, 144–151 (1995).
Yoshida, H., Yamamoto, H., Uchiumi, T. & Wada, A. RMF inactivates ribosomes by covering the peptidyl transferase centre and entrance of peptide exit tunnel. Genes Cells 9, 271–278 (2004).
Cagliero, C. & Jin, D. J. Dissociation and re-association of RNA polymerase with DNA during osmotic stress response in Escherichia coli. Nucleic Acids Res. 41, 315–326 (2013).
McGary, K. & Nudler, E. RNA polymerase and the ribosome: the close relationship. Curr. Opin. Microbiol. 16, 112–117 (2013).
Ray-Soni, A., Bellecourt, M. J. & Landick, R. Mechanisms of bacterial transcription termination: all good things must end. Annu. Rev. Biochem. 85, 319–347 (2016).
Morita, R. Y. Bacteria in Oligotrophic Environments: Starvation-Survival Lifestyle (Chapman & Hall, London, 1997).
Reeve, C. A., Amy, P. S. & Matin, A. Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J. Bacteriol. 160, 1041–1046 (1984).
Houser, J. R. et al. Controlled measurement and comparative analysis of cellular components in E. coli reveals broad regulatory changes in response to glucose starvation. PLoS Comput. Biol. 11, e1004400 (2015).
Phaiboun, A., Zhang, Y., Park, B. & Kim, M. Survival kinetics of starving bacteria is biphasic and density-dependent. PLoS Comput. Biol. 11, e1004198 (2015).
Iyer, S., Park, B. R. & Kim, M. Absolute quantitative measurement of transcriptional kinetic parameters in vivo. Nucleic Acids Res. 44, e142 (2016).
Garcia, H. G., Lee, H. J., Boedicker, J. Q. & Phillips, R. Comparison and calibration of different reporters for quantitative analysis of gene expression. Biophys. J. 101, 535–544 (2011).
Bremer, H. & Dennis, P. Modulation of chemical composition and other parameters of the cell at different exponential growth rates. EcoSal Plus https://doi.org/10.1128/ecosal.5.2.3 (2008).
Proshkin, S., Rahmouni, A. R., Mironov, A. & Nudler, E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 328, 504–508 (2010).
Kepes, A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Progress. Biophys. Mol. Biol. 19, 199–236 (1969).
Schleif, R., Hess, W., Finkelstein, S. & Ellis, D. Induction kinetics of the l-arabinose operon of Escherichia coli. J. Bacteriol. 115, 9–14 (1973).
Jin, D. J., Burgess, R. R., Richardson, J. P. & Gross, C. A. Termination efficiency at rho-dependent terminators depends on kinetic coupling between RNA polymerase and rho. Proc. Natl Acad. Sci. USA 89, 1453–1457 (1992).
Dai, X. et al. Reduction of translating ribosomes enables Escherichia coli to maintain elongation rates during slow growth. Nat. Microbiol 2, 16231 (2016).
Komissarova, N. & Kashlev, M. RNA polymerase switches between inactivated and activated states by translocating back and forth along the DNA and the RNA. J. Biol. Chem. 272, 15329–15338 (1997).
Nudler, E., Mustaev, A., Goldfarb, A. & Lukhtanov, E. The RNA–DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell 89, 33–41 (1997).
Mamata, S. & Stefan, K. Backtracking dynamics of RNA polymerase: pausing and error correction. J. Phys. Condens. Matter 25, 374104 (2013).
Steitz, T. A. A structural understanding of the dynamic ribosome machine. Nat. Rev. Mol. Cell Biol. 9, 242–253 (2008).
Zhang, Y. et al. DksA guards elongating RNA polymerase against ribosome-stalling-induced arrest. Mol. Cell 53, 766–778 (2014).
Potrykus, K. & Cashel, M. (p)ppGpp: still magical? Annu. Rev. Microbiol. 62, 35–51 (2008).
Villadsen, I. S. & Michelsen, O. Regulation of PRPP and nucleoside tri and tetraphosphate pools in Escherichia coli under conditions of nitrogen starvation. J. Bacteriol. 130, 136–143 (1977).
Metzger, S., Schreiber, G., Aizenman, E., Cashel, M. & Glaser, G. Characterization of the relA1 mutation and a comparison of relA1 with new relA null alleles in Escherichia coli. J. Biol. Chem. 264, 21146–21152 (1989).
Gentry, D. R. & Cashel, M. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol. Microbiol. 19, 1373–1384 (1996).
Ryals, J., Little, R. & Bremer, H. Control of rRNA and tRNA syntheses in Escherichia coli by guanosine tetraphosphate. J. Bacteriol. 151, 1261–1268 (1982).
Cashel, M. & Gallant, J. Two compounds implicated in the function of the RC gene of Escherichia coli. Nature 221, 838–841 (1969).
Spira, B., Silberstein, N. & Yagil, E. Guanosine 3’,5’-bispyrophosphate (ppGpp) synthesis in cells of Escherichia coli starved for Pi. J. Bacteriol. 177, 4053–4058 (1995).
Kingston, R. E., Nierman, W. C. & Chamberlin, M. J. A direct effect of guanosine tetraphosphate on pausing of Escherichia coli RNA polymerase during RNA chain elongation. J. Biol. Chem. 256, 2787–2797 (1981).
Kingston, R. E. & Chamberlin, M. J. Pausing and attenuation of in vitro transcription in the rrnB operon of E. coli. Cell 27, 523–531 (1981).
Krohn, M. & Wagner, R. Transcriptional pausing of RNA polymerase in the presence of guanosine tetraphosphate depends on the promoter and gene sequence. J. Biol. Chem. 271, 23884–23894 (1996).
Vogel, U., Sørensen, M., Pedersen, S., Jensen, K. F. & Kilstrup, M. Decreasing transcription elongation rate in Escherichia coli exposed to amino acid starvation. Mol. Microbiol. 6, 2191–2200 (1992).
Sørensen, M. A., Jensen, K. F. & Pedersen, S. High concentrations of ppGpp decrease the RNA chain growth rate. J. Mol. Biol. 236, 441–454 (1994).
van Ooyen, A. J. J., Gruber, M. & Jørgensen, P. The mechanism of action of ppGpp on rRNA synthesis in vitro. Cell 8, 123–128 (1976).
Barker, M. M., Gaal, T., Josaitis, C. A. & Gourse, R. L. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol. 305, 673–688 (2001).
Travers, A. A. Promoter sequence for stringent control of bacterial ribonucleic acid synthesis. J. Bacteriol. 141, 973–976 (1980).
Ross, W. et al. ppGpp binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol. Cell 62, 811–823 (2016).
Roghanian, M., Zenkin, N. & Yuzenkova, Y. Bacterial global regulators DksA/ppGpp increase fidelity of transcription. Nucleic Acids Res. 43, 1529–1536 (2015).
Svitil, A. L., Cashel, M. & Zyskind, J. W. Guanosine tetraphosphate inhibits protein synthesis in vivo. A possible protective mechanism for starvation stress in Escherichia coli. J. Biol. Chem. 268, 2307–2311 (1993).
Xiao, H. et al. Residual guanosine 3',5'-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266, 5980–5990 (1991).
Harinarayanan, R., Murphy, H. & Cashel, M. Synthetic growth phenotypes of Escherichia coli lacking ppGpp and transketolase A (tktA) are due to ppGpp-mediated transcriptional regulation of tktB. Mol. Microbiol. 69, 882–894 (2008).
Haugen, S. P., Ross, W. & Gourse, R. L. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Micro. 6, 507–519 (2008).
Durfee, T., Hansen, A.-M., Zhi, H., Blattner, F. R. & Jin, D. J. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 190, 1084–1096 (2008).
Brown, D. R., Barton, G., Pan, Z., Buck, M. & Wigneshweraraj, S. Nitrogen stress response and stringent response are coupled in Escherichia coli. Nat. Commun. 5, 4115 (2014).
Soupene, E. et al. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185, 5611–5626 (2003).
Lyons, E., Freeling, M., Kustu, S. & Inwood, W. Using genomic sequencing for classical genetics in E. coli K12. PLoS ONE 6, e16717 (2011).
Brown, S. D. & Jun, S. Complete genome sequence of Escherichia coli NCM3722. Genome Announc. 3 (2015).
Csonka, L. N., Ikeda, T. P., Fletcher, S. A. & Kustu, S. The accumulation of glutamate is necessary for optimal growth of Salmonella typhimurium in media of high osmolality but not induction of the proU operon. J. Bacteriol. 176, 6324–6333 (1994).
Kim, M., Zhang, Z. G., Okano, H., Yan, D. L., Groisman, A. & Hwa, T. Need-based activation of ammonium uptake in Escherichia coli. Mol. Syst. Biol. 8, 616 (2012).
Novick, A. & Weiner, M. Enzyme induction as an all-or-none phenomenon. Proc. Natl Acad. Sci. USA 43, 553–566 (1957).
Kuhlman, T., Zhang, Z., Saier, M. H. Jr & Hwa, T. Combinatorial transcriptional control of the lactose operon of Escherichia coli. Proc. Natl Acad. Sci. USA 104, 6043–6048 (2007).
Miller, J. H. Experiments in Molecular Genetics (Cold Spring Harbor Laboratory Press, New York, 1972).
Dieterich, D. C., Link, A. J., Graumann, J., Tirrell, D. A. & Schuman, E. M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl Acad. Sci. USA 103, 9482–9487 (2006).
Beatty, K. E. et al. Live-cell imaging of cellular proteins by a strain-promoted azide–alkyne cycloaddition. ChemBioChem 11, 2092–2095 (2010).
Hatzenpichler, R. et al. In situ visualization of newly synthesized proteins in environmental microbes using amino acid tagging and click chemistry. Environ. Microbiol. 16, 2568–2590 (2014).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Merril, C. R. Gel-staining techniques. Methods Enzymol. 182, 477–488 (1990).
Acknowledgements
We thank M. Cashel, R.L. Gourse and J. Wang for helpful discussions and kindly sharing strains with us. This work was funded by Research Corporation for Science Advancement (24097) and the Human Frontier Science Program (RGY0072/2015).
Author information
Authors and Affiliations
Contributions
S.I., D.L., B.R.P. and M.K. designed the study. S.I., D.L. and B.R.P. performed experiments. S.I. D.K. and B.R.P. analysed the data. M.K. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Supplementary Figures 1–22, Supplementary Tables 1–5, Supplementary References.
Rights and permissions
About this article
Cite this article
Iyer, S., Le, D., Park, B.R. et al. Distinct mechanisms coordinate transcription and translation under carbon and nitrogen starvation in Escherichia coli. Nat Microbiol 3, 741–748 (2018). https://doi.org/10.1038/s41564-018-0161-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0161-3
This article is cited by
-
Co-transcriptional gene regulation in eukaryotes and prokaryotes
Nature Reviews Molecular Cell Biology (2024)
-
Stringent response ensures the timely adaptation of bacterial growth to nutrient downshift
Nature Communications (2023)
-
Dissociation kinetics of small-molecule inhibitors in Escherichia coli is coupled to physiological state of cells
Communications Biology (2023)
-
ppGpp accumulation reduces the expression of the global nitrogen homeostasis-modulating NtcA regulon by affecting 2-oxoglutarate levels
Communications Biology (2023)
-
Maintenance of tRNA and elongation factors supports T3SS proteins translational elongations in pathogenic bacteria during nutrient starvation
Cell & Bioscience (2022)