Abstract
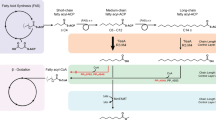
Microorganisms can catabolize a wide range of organic compounds and therefore have the potential to perform many industrially relevant bioconversions. One barrier to realizing the potential of biorefining strategies lies in our incomplete knowledge of metabolic pathways, including those that can be used to assimilate naturally abundant or easily generated feedstocks. For instance, levulinic acid (LA) is a carbon source that is readily obtainable as a dehydration product of lignocellulosic biomass and can serve as the sole carbon source for some bacteria. Yet, the genetics and structure of LA catabolism have remained unknown. Here, we report the identification and characterization of a seven-gene operon that enables LA catabolism in Pseudomonas putida KT2440. When the pathway was reconstituted with purified proteins, we observed the formation of four acyl-CoA intermediates, including a unique 4-phosphovaleryl-CoA and the previously observed 3-hydroxyvaleryl-CoA product. Using adaptive evolution, we obtained a mutant of Escherichia coli LS5218 with functional deletions of fadE and atoC that was capable of robust growth on LA when it expressed the five enzymes from the P. putida operon. This discovery will enable more efficient use of biomass hydrolysates and metabolic engineering to develop bioconversions using LA as a feedstock.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pileidis, F. D. & Titirici, M. M. Levulinic acid biorefineries: new challenges for efficient utilization of biomass. ChemSusChem 9, 562–582 (2016).
Bozell, J. J. et al. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl. 28, 227–239 (2000).
Werpy, T. & Petersen, G. Top Value Added Chemicals from Biomass (US Department of Energy, 2004); http://www.pnl.gov/main/publications/external/technical_reports/PNNL-14808.pdf.
Lange, J. P. et al. Valeric biofuels: a platform of cellulosic transportation fuels. Angew. Chem. Int. Ed. 49, 4479–4483 (2010).
Alonso, D. M., Wettstein, S. G. & Dumesic, J. A. Gamma-valerolactone, a sustainable platform molecule derived from lignocellulosic biomass. Green Chem. 15, 584–595 (2013).
Joshi, H., Moser, B. R., Toler, J., Smith, W. F. & Walker, T. Ethyl levulinate: a potential bio-based diluent for biodiesel which improves cold flow properties. Biomass Bioenergy 35, 3262–3266 (2011).
Zhang, Z., Dong, K. & Zhao, Z. Efficient conversion of furfuryl alcohol into alkyl levulinates catalyzed by an organic-inorganic hybrid solid acid catalyst. ChemSusChem 4, 112–118 (2011).
Demolis, A., Essayem, N. & Rataboul, F. Synthesis and applications of alkyl levulinates. ACS Sustain. Chem. Eng. 2, 1338–1352 (2014).
Moore, J. A. & Tannahill, T. Homo- and co-polycarbonates and blends derived from diphenolic acid. High Perform. Polym. 13, 305–316 (2001).
Guo, Y., Li, K., Yu, X. & Clark, J. H. Mesoporous H3PW12O40-silica composite: efficient and reusable solid acid catalyst for the synthesis of diphenolic acid from levulinic acid. Appl. Catal. B 81, 182–191 (2008).
Chung, S. H., Choi, G. G., Kim, H. W. & Rhee, Y. H. Effect of levulinic acid on the production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Ralstonia eutropha KHB-8862. Society 39, 79–82 (2001).
Berezina, N. & Yada, B. Improvement of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by dual feeding with levulinic acid and sodium propionate in Cupriavidus necator. Nat. Biotechnol. 33, 231–236 (2016).
Jang, J. H. & Rogers, P. L. Effect of levulinic acid on cell growth and poly-beta-hydroxyalkanoate production by Alcaligenes sp SH-69. J. Chem. Inf. Model. 18, 219–224 (1996).
Habe, H. et al. Bacterial production of short-chain organic acids and trehalose from levulinic acid: a potential cellulose-derived building block as a feedstock for microbial production. Bioresour. Technol. 177, 381–386 (2015).
Martin, C. H., Wu, D., Prather, K. L. J. & Jones Prather, K. L. Integrated bioprocessing for the pH-dependent production of 4-valerolactone from levulinate in Pseudomonas putida KT2440. Appl. Environ. Microbiol. 76, 417–424 (2010).
Yeon, Y. J., Park, H. Y. & Yoo, Y. J. Enzymatic reduction of levulinic acid by engineering the substrate specificity of 3-hydroxybutyrate dehydrogenase. Bioresour. Technol. 134, 377–380 (2013).
Jaremko, M. & Yu, J. The initial metabolic conversion of levulinic acid in Cupriavidus necator. J. Biotechnol. 155, 293–298 (2011).
Martin, C. H. & Prather, K. L. J. High-titer production of monomeric hydroxyvalerates from levulinic acid in Pseudomonas putida. J. Biotechnol. 139, 61–67 (2009).
Zhang, G. F. et al. Catabolism of 4-hydroxyacids and 4-hydroxynonenal via 4-hydroxy-4-phosphoacyl-CoAs. J. Biol. Chem. 284, 33521–33534 (2009).
Harris, S. R. et al. Metabolism of levulinate in perfused rat livers and live rats: conversion to the drug of abuse 4-hydroxypentanoate. J. Biol. Chem. 286, 5895–5904 (2011).
Martínez-García, E., Calles, B., Arévalo-Rodríguez, M. & de Lorenzo, V. pBAM1: an all-synthetic genetic tool for analysis and construction of complex bacterial phenotypes. BMC Microbiol. 11, 38 (2011).
Wetmore, K. M. M. et al. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. mBio 6, e00306-15 (2015).
Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990).
Schramm, G., Bruchhaus, I. & Roeder, T. A simple and reliable 5′-RACE approach. Nucleic Acids Res. 28, E96 (2000).
Espah Borujeni, A., Channarasappa, A. S. S. & Salis, H. M. M. Translation rate is controlled by coupled trade-offs between site accessibility, selective RNA unfolding and sliding at upstream standby sites. Nucleic Acids Res. 42, 2646–2659 (2014).
Barrios, H., Valderrama, B. & Morett, E. Compilation and analysis of σ54-dependent promoter sequences. Nucleic Acids Res. 27, 4305–4313 (1999).
Fox, J. D., Routzahn, K. M., Bucher, M. H. & Waugh, D. S. Maltodextrin-binding proteins from diverse bacteria and archaea are potent solubility enhancers. FEBS Lett. 537, 53–57 (2003).
Striebel, F. et al. Bacterial ubiquitin-like modifier Pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nat. Struct. Mol. Biol. 16, 647–651 (2009).
Yamamoto, S. & Kutsukake, K. FliT acts as an anti-FlhD2C2 factor in the transcriptional control of the flagellar regulon in Salmonella enterica serovar Typhimurium. J. Bacteriol. 188, 6703–6708 (2006).
Dijkman, W. P. & Fraaije, M. W. Discovery and characterization of a 5-hydroxymethylfurfural oxidase from Methylovorus sp. strain MP688. Appl. Environ. Microbiol. 80, 1082–1090 (2014).
Simons, R. W., Egan, P. A., Chute, H. T. & Nunn, W. D. Regulation of fatty acid degradation in Escherichia coli: isolation and characterization of strains bearing insertion and temperature-sensitive mutations in gene fadR. 142, 621–632 (1980).
Brock, M., Maerker, C., Schutz, A., Volker, U. & Buckel, W. Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur. J. Biochem. 269, 6184–6194 (2002).
Man, W. J., Li, Y., O’Connor, C. D. & Wilton, D. C. The binding of propionyl-CoA and carboxymethyl-CoA to Escherichia coli citrate synthase. Biochim. Biophys. Acta 1250, 69–75 (1995).
Jiang, W., Bikard, D., Cox, D., Zhang, F. & Marraffini, L. A. RNA-guided editing of bacterial genomes using CRISPR-Cas systems. Nat. Biotechnol. 31, 233–239 (2013).
Jiang, Y. et al. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl. Environ. Microbiol. 81, 2506–2514 (2015).
Li, Y. et al. Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab. Eng. 31, 13–21 (2015).
Moreno, R., Marzi, S., Romby, P. & Rojo, F. The Crc global regulator binds to an unpaired A-rich motif at the Pseudomonas putida alkS mRNA coding sequence and inhibits translation initiation. Nucleic Acids Res. 37, 7678–7690 (2009).
Moreno, R., Martínez-Gomariz, M., Yuste, L., Gil, C. & Rojo, F. The Pseudomonas putida Crc global regulator controls the hierarchical assimilation of amino acids in a complete medium: evidence from proteomic and genomic analyses. Proteomics 9, 2910–2928 (2009).
Storz, G., Wolf, Y. I. & Ramamurthi, K. S. Small proteins can no longer be ignored. Annu. Rev. Biochem. 83, 753–777 (2014).
Su, M., Ling, Y., Yu, J., Wu, J. & Xiao, J. Small proteins: untapped area of potential biological importance. Front. Genet. 4, 286 (2013).
Felnagle, E. A. et al. MbtH-like proteins as integral components of bacterial nonribosomal peptide synthetases. Biochemistry 49, 8815–8817 (2010).
Baltz, R. H. Function of MbtH homologs in nonribosomal peptide biosynthesis and applications in secondary metabolite discovery. J. Ind. Microbiol. Biotechnol. 38, 1747–1760 (2011).
Gräwert, T. et al. Structure of active IspH enzyme from Escherichia coli provides mechanistic insights into substrate reduction. Angew. Chem. Int. Ed. 48, 5756–5759 (2009).
Hecht, S. et al. Studies on the nonmevalonate pathway to terpenes: the role of the GcpE (IspG) protein. Proc. Natl Acad. Sci. USA 98, 14837–14842 (2001).
Jenkins, L. S. & Nunn, W. D. Genetic and molecular characterization of the genes involved in short-chain fatty acid degradation in Escherichia coli: the ato system. J. Bacteriol. 169, 42–52 (1987).
Matta, M. K., Lioliou, E. E., Panagiotidis, C. H., Kyriakidis, D. A. & Panagiotidis, C. A. Interactions of the antizyme AtoC with regulatory elements of the Escherichia coli atoDAEB operon. J. Bacteriol. 189, 6324–6332 (2007).
Campbell, J. W. & Cronan, J. E. J. The enigmatic Escherichia coli fadE gene is yafH. J. Bacteriol. 184, 3759–3764 (2002).
Daley, D. O. et al. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308, 1321–1323 (2005).
Díaz-Mejía, J. J., Babu, M. & Emili, A. Computational and experimental approaches to chart the Escherichia coli cell-envelope-associated proteome and interactome. FEMS Microbiol. Rev. 33, 66–97 (2009).
Jenkins, L. S. & Nunn, W. D. Regulation of the ato operon by the atoC gene in Escherichia coli. J. Bacteriol. 169, 2096–2102 (1987).
Gibson, D. G., Young, L., Chuang, R.-Y., Venter, J. C., Hutchison, C. A. & Smith, H. O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Sambrook, J., Russell, D. W. & David, W. Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Laboratory Press, New York, 2001).
Neidhardt, F. C., Bloch, P. L. & Smith, D. F. Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 (1974).
Wetmore, K. M. et al. Rapid quantification of mutant fitness in diverse bacteria by sequencing randomly bar-coded transposons. mBio 6, e00306–15 (2015).
Dehal, P. S. et al. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res. 38, 396–400 (2009).
Pinto, F. L., Thapper, A., Sontheim, W. & Lindblad, P. Analysis of current and alternative phenol based RNA extraction methodologies for cyanobacteria. BMC Mol. Biol. 10, 79 (2009).
Schäfer, A., Tauch, A., Jäger, W., Kalinowski, J., Thierbach, G. & Pühler, A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145, 69–73 (1994).
Graf, N. & Altenbuchner, J. Development of a method for markerless gene deletion in Pseudomonas putida. Appl. Environ. Microbiol. 77, 5549–5552 (2011).
Cock, P. J. A. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Li, H. et al. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Goto, H. et al. Dynamics of mitochondrial heteroplasmy in three families investigated via a repeatable re-sequencing study. Genome Biol. 12, R59 (2011).
Li, Y. et al. Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab. Eng. 31, 13–21 (2015).
Qi, L. S. et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–1183 (2013).
Acknowledgements
Work in the Pfleger laboratory was funded by the National Science Foundation (CBET-114678) and the William F. Vilas Trust. Work in the Deutschbauer and Arkin laboratories was funded by ENIGMA, a Scientific Focus Area Program, supported by the US Department of Energy, Office of Science, Office of Biological and Environmental Research and Genomics: GTLFoundational Science through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the US Department of Energy. Work in the Amador-Noguez laboratory was funded by the HHMI International Student Research Fellowship. R.L.C. was supported by the NIH NHGRI Genomic Sciences Training Program (T32 HG002760). A.L.M. was supported by an NSF SEES fellowship (GEO-1215871). J.M.R. was supported by an NSF Graduate Research Fellowship (DGE-1256259).
The authors thank J. Escalante for providing plasmid pK18mobsacB and J. Altenbuchner for providing strain P. putida KTU and plasmid pJOE6261.2. The authors acknowledge the Mass Spectrometry/Proteomics Facility at the UW–Madison Biotechnology Center for performing the in-gel digest and providing the LC–MS/MS results, and the UW–Madison Biotechnology Center DNA Sequencing Facility for providing genomic sequencing services. The authors also thank G. Gordon for help with the analysis of the E. coli genomic sequencing single nucleotide polymorphisms (SNPs).
Author information
Authors and Affiliations
Contributions
J.M.R., D.E.A. and B.F.P. conceived the study. J.M.R. designed and performed the experiments and analysed the data, with the following exceptions. T.P. and D.A.-N. designed the LC–MS/MS experiments and T.P. performed the LC–MS and LC–MS/MS experiments. D.E.A. and J.M.T. performed the transposon library screen. C.E.C. assisted with the promoter and CoA ligase assay. A.L.M. proposed, and helped design and perform, the pulldown experiment. Y.S. and J.R. prepared the RB-TnSeq mutant library of P. putida KT2440 (Putida_ML5). K.M.W., R.L.C., J.R. and A.M.D. performed the fitness assays with the Putida_ML5 library. M.N.P. performed the data analysis to determine fitness values. R.L.C. prepared the supplementary analysis of the Putida_ML5 fitness experiments. A.M.D. and A.P.A. managed the Bar-Seq experiments. C.R.M helped design and analyse the prevalence of the lva operon in other organisms. J.M.R. and B.F.P. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary Information
Supplementary Notes, Supplementary References, Supplementary Figures 1–6, Supplementary Tables 1–4, Supplementary Tables 7–10.
Supplementary Table 5 and 6
Species with LvaABCD homologues and Species with LvaACD homologues.
Supplementary Data Set 1
Data code.
Rights and permissions
About this article
Cite this article
Rand, J.M., Pisithkul, T., Clark, R.L. et al. A metabolic pathway for catabolizing levulinic acid in bacteria. Nat Microbiol 2, 1624–1634 (2017). https://doi.org/10.1038/s41564-017-0028-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-017-0028-z
This article is cited by
-
Biodegradation of furfuryl alcohol by indigenous Bacillus species of industrial effluent-contaminated sites: estimation, biokinetics and toxicity assessment of bio-transformed metabolites
World Journal of Microbiology and Biotechnology (2024)
-
Enhancing translation efficiency and exploring constraints in high-level 4-hydroxyvaleric acid production from levulinic acid in Escherichia coli
Systems Microbiology and Biomanufacturing (2024)
-
Biosynthesis of cannabinoid precursor olivetolic acid in genetically engineered Yarrowia lipolytica
Communications Biology (2022)
-
Inducible and tunable gene expression systems for Pseudomonas putida KT2440
Scientific Reports (2021)
-
Investigation of Bar-seq as a method to study population dynamics of Saccharomyces cerevisiae deletion library during bioreactor cultivation
Microbial Cell Factories (2020)