Abstract
RNA-based therapeutics have shown tremendous promise in disease intervention at the genetic level, and some have been approved for clinical use, including the recent COVID-19 messenger RNA vaccines. The clinical success of RNA therapy is largely dependent on the use of chemical modification, ligand conjugation or non-viral nanoparticles to improve RNA stability and facilitate intracellular delivery. Unlike molecular-level or nanoscale approaches, macroscopic hydrogels are soft, water-swollen three-dimensional structures that possess remarkable features such as biodegradability, tunable physiochemical properties and injectability, and recently they have attracted enormous attention for use in RNA therapy. Specifically, hydrogels can be engineered to exert precise spatiotemporal control over the release of RNA therapeutics, potentially minimizing systemic toxicity and enhancing in vivo efficacy. This Review provides a comprehensive overview of hydrogel loading of RNAs and hydrogel design for controlled release, highlights their biomedical applications and offers our perspectives on the opportunities and challenges in this exciting field of RNA delivery.
Similar content being viewed by others
Main
Nucleic-acid-based therapies, such as DNA, antisense oligonucleotides (ASOs), small interfering RNAs (siRNA) and messenger RNAs (mRNA), have been widely used in diverse biomedical applications. As a type of nucleic acid essential for all known life, RNA molecules play numerous regulatory roles, such as instructing protein expression and modulating targeted genes1,2,3. So far, several RNA therapeutics, mainly siRNA and mRNA, have been clinically approved for different diseases (Table 1), with many others in clinical trials. mRNA helps the body make its own missing, defective or functional exogenous proteins (for example, antigens)4, while siRNA reduces the expression of endogenously expressed proteins or pathological proteins5. Additionally, microRNAs (miRNAs) and other non-coding RNAs have also been explored for regulating gene expression at the post-transcriptional level6.
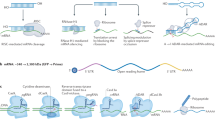
Despite the considerable therapeutic potential of RNAs, limitations on their in vivo delivery have been reported, including enzymatic susceptibility, extracellular and cellular barriers, and difficulties in trafficking to the subcellular compartment where the cargo will be active1. Therefore, the majority of clinical-stage RNA therapies are based on chemical modification (for example, phosphorothioate linkage), ligand conjugation (for example, N-acetylgalactosamine (GalNAC)) or non-viral nanoparticle (NP) delivery (for example, lipid NP)7. Specifically, chemical modification improves enzymatic and metabolic stability8, and ligand conjugation improves delivery to specific organs and cell types9. Finally, NPs protect encapsulated RNA and improve pharmacokinetics and endosomal escape10. However, these delivery methods have their own limitations, with further improvements needed for transfection efficiency11, organ/cell delivery specificity12, RNA stability13 and circumventing immune activation14, which may require the development of entirely different categories of delivery systems. Along these lines, substantial efforts have recently been made to explore the use of macroscale hydrogels for RNA-based therapeutics delivery, as well as a variety of biomedical applications ranging from gene silencing and protein replacement to immunomodulation (Fig. 1)15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40.
Coloured boxes indicate the type of biomedical application: cancer therapy (orange), bone regeneration (blue), immunomodulation (yellow), cardiac repair (red) and angiogenesis (grey). ACpG-STAT3, cytosine-phosphorothioate-guanine-signal transducer and activator of transcription 3; DextranVS, dextran vinylsulfone; GelMA, gelatin methacryloyl; HP-HA-PEG, a thiol-modified analogue of heparin-thiol-modified hyaluronan-poly(ethylene glycol) diacrylate; hyd, hydrogel; IL, interleukin; MPEG, methoxypolyethylene glycol; mTOR, mammalian target of rapamycin; PAA, polyacrylamide; PCL, poly(ε-caprolactone); PE, polyethylene; PEG4SH, tetra-thiolated polyethyleneglycol; PEI-DA, deoxycholic acid-modified polyethylenimine polymeric conjugates; PLA-DX-PEG, poly-d,l-lactic acid-p-dioxanone-polyethylene glycol block co-polymer; PLK, serine/threonine-protein kinase; Rb1/Meis2, retinoblastoma1/meis homeobox 2; RGM, RNA gene for miRNAs; SPARC, secreted protein acidic and rich in cysteine15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40.
Hydrogels are composed of a water-swollen three-dimensional network that recapitulates the intrinsic properties of the native extracellular matrix (ECM), making them useful for applications in tissue engineering, drug delivery, cellular morphogenesis, among others41,42. The unique physicochemical features of hydrogels allow the maintenance of RNA biological activity, the retention and sustained release of RNA as local delivery carriers (for example, injectable systems), and the delivery of high concentrations of payloads to a target site in an on-demand/pulsatile manner via stimuli-responsive strategies (Fig. 2)43,44. Thus, hydrogels could improve RNA stability, reduce unnecessary loss of therapeutics associated with systemic delivery, mitigate undesirable off-target toxicities and avoid the necessity of multiple doses. All of these make hydrogels an appealing system for RNA-based therapeutics delivery, complementary to the clinical-stage platforms mentioned above.
Hydrogels provide a unique strategy for local administration of RNA, overcoming some of the difficulties associated with systemic RNA delivery. They enable a localized, controlled and sustained delivery of high levels of payloads, while maintaining RNA biological activity. Off-target effects and the need for multiple payload administrations in systemic delivery may thus be avoided.
Compelling evidence supporting the application of hydrogels for delivery of RNAs and other nucleic acids has been gathered ever since the first use of polymer pellets for sustained nucleic acid release in 197645. This Review aims to summarize RNA-loading strategies in hydrogels, discuss the design of hydrogels for controlled delivery of RNAs, highlight recent progress in biomedical applications, and provide perspectives on the challenges and opportunities in this blossoming research field.
RNA loading in hydrogels
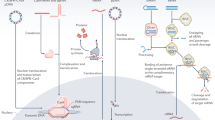
RNA can be loaded into hydrogels either by direct inclusion of the naked RNA or by encapsulating RNA nanocarriers (Fig. 3). The loading of naked RNA largely depends on the physicochemical interactions between the RNA and the hydrogel network. As a comparison, nanocarriers may offer improved RNA bioactivity, better controllability of RNA release and targeting of specific cells, while the loading is dependent on the interactions of nanocarriers with the hydrogel.
a, RNA is loaded into hydrogel either with no manipulation (naked RNA) or by means of nanocarriers. b, RNA-loaded hydrogels can be used as implantable scaffolds or as injectable gels for local RNA delivery. The fine-tunable physical, biochemical and biological features of hydrogels allow the sustained and/or controllable release of RNA. Upon cellular entry (for example, via naked RNA or RNA-loaded nanocarriers), RNA reaches the proper subcellular compartment to initiate protein production/inhibition.
Naked RNAs
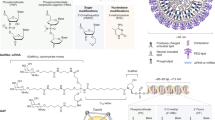
Multiple strategies have been developed for loading naked RNAs into hydrogels, such as electrostatic interaction, covalent conjugation, host–guest interaction or combinations thereof (Fig. 4). Here, we introduce each strategy and the associated hydrogels, and discuss their features for delivery of naked RNA.
RNA therapeutics can interact with hydrogel networks through ionic bonds between negatively charged RNA parts and positively charged hydrogel network parts; hydrogen bonds produced when positively charged hydrogen atoms come within a certain radius of an electronegative acceptor atom; covalent bonds that chemically link the RNA to polymer hydrogel chains; hydrophobic interactions that use modified RNA; and non-specific interactions.
Ionic bond
Cationic/ionizable polymers and lipids, the most widely studied non-viral delivery materials, can interact with negatively charged biomolecules, making ionic bonds a simple and robust method for the encapsulation of RNAs and other nucleic acids in hydrogels46. However, synthetic polycations may cause moderate-to-high toxicity, mainly due to their high positive charge. Further, synthetic cationic polymers generally have a low molecular weight and a highly branched structure, which may limit their potential applications in hydrogel formation. Thus, conjugation of synthetic and natural polycations to hydrogels or the use of hydrogels solely based on natural polycations emerged to address these concerns37,47. For example, several different biodegradable polymers and fabrication systems were studied for localized delivery of naked siRNA: calcium crosslinked alginate, photocrosslinked alginate and acid-solubilized collagen47. siRNA was rapidly released within a week from the highly negatively charged alginate hydrogels, but required over two weeks to be released from the collagen hydrogel due to the effect of the amine groups. The incorporation of positively charged polyethylenimine (PEI) or chitosan further delayed siRNA release. Moreover, ionic bonds are susceptible to pH changes, which may hinder the sustained release of the loaded biomolecules. Factors such as the number and type of charge groups (for example, primary, secondary and quaternary amino groups and amidine groups) per single polymer can also help determine the ultimate release profile.
Hydrogen bond
Neutrally charged polymers such as polyvinyl alcohol (PVA) can bind to nucleic acids through the interaction of the positive hydrogen atom, which establishes an electrostatic link with electronegative acceptor atoms48. Such polymers can be further modified with certain chemical moieties to increase intermolecular hydrogen bonding interactions between the hydrogel and the RNAs. Similarly, negatively charged polysaccharides such as alginate and hyaluronic acid (HA) have been explored to promote the controlled release of RNAs, which can also be modified with additional moieties to increase the number of hydrogen bonds to the encapsulated nucleic acids. For example, HA-PVA hydrogels were shown to release siRNA in vitro more slowly than PVA hydrogels, which was attributed to a higher number of hydrogen bonds between the siRNA and the HA backbone49. It is important to emphasize that hydrogen bonds are primarily weak electrostatic interactions, which may not induce strong binding of RNAs to the hydrogels. Consequently, hydrogen and ionic bonds are sometimes combined to boost interactions between the hydrogel and RNAs, through the addition of cationic molecules or polymers to the hydrogel formula39,50.
Covalent bond
Covalent conjugation of RNAs to the backbone of hydrogels allows for the homogeneous and predictable distribution of a large quantity of RNAs with minimal initial burst release. Although this method is very common in small-molecule drug delivery, there are very few examples of covalently binding RNAs51. For instance, siRNA was covalently tethered to the photocrosslinked dextran hydrogels via Michael-addition chemistry. Upon hydrolytic degradation of ester and/or disulfide linkages, the profile release of siRNA was prolonged up to 10 days in comparison with the unbound compound that was released in the first 12 hours. By tailoring the degradable linkages or the amount of tethered siRNA in the hydrogel network is possible to control the cargo amount and its profile release. However, the conjugation of siRNA into the hydrogel network possesses additional technical complexity.
Hydrophobic interaction
Hydrophobic interactions occur through the formation of a clathrate cage, an ice-like matrix of water molecules formed through hydrogen bonds, around the hydrophobe52. The host–guest pairs are relatively easy to synthesize and can interact with biological molecules, being typically driven by molecule size and hydrophobicity. Guest–host pairs are widely used for injectable hydrogel fabrication mainly due to their dynamic bonds that, upon, can reform44. For example, cyclodextrin (CD) showed relatively low toxicity and high water solubility29, enabling a range of hydrophobic guest molecules to be embedded in their interior cavities53. HA was modified with CD, as a host, or with adamantane, as a guest, which were self-assembled into injectable hydrogels. The HA assembly system enables the formation of complex CD-cholesterol-modified miR-302 interactions for miRNA local and sustained release40. The release of miRNA from the HA hydrogel network is measured over three weeks (in vitro studies), and it is faster than the erosion of the hydrogel. Thus, it is hypothesized that miRNAs diffuse from the hydrogel network, highlighting the crucial role of the anionic repulsion of HA to miRNA and the hydrogel guest–host interactions.
As an alternative approach, supramolecular hydrogels can also be formed by self-assembly of biocompatible small-molecule hydrogelators, which is triggered by intramolecular π–π stacking of hydrogelator molecules and RNAs. For example, polyethylene glycol (PEG) modified with ureido-pyrimidinone moieties (UPy-PEG) was able to dimerize in water, producing a fibrous supramolecular hydrogel54. siRNA and miRNA were covalently conjugated with cholesterol to directly interact with the hydrophobic core of the fibre, which fine-tunes siRNA and miRNA profile release. Otherwise, supramolecular hydrogelator molecules (for example, tetrazole and spiropyran) can be added to facilitate hydrophobic interactions with the hydrophobic side chains of amino acids within the encapsulated RNA structures55. However, in these hydrogels the ratio of hydrophobic moieties to hydrophilic regions must be adjusted carefully to preserve water-uptake capacity. It should also be noted that such hydrophobic interactions do not depend on RNA itself but on the hydrophobic modifier on RNA.
Non-specific interaction
In some cases, RNA loading in hydrogels is merely mediated by non-specific interactions. The RNA release may therefore be governed simply by diffusion-controlled mechanisms, which are discussed in detail in the following section. For instance, one could increase the crosslinking density and consequently decrease the swelling and drug release rate, or reduce the macromolecular mesh size by altering the macromolecular structure and therefore extend the drug release time. This is often the case for thermo-sensitive hydrogels, such as poly(N-isopropylacrylamide)56, which can be formed upon in vivo administration, instigated by the sol-to-gel phase transition. A major limitation associated with thermohydrogels is their lack of biodegradability, which could be addressed by copolymerizing them with biodegradable polymers. The limited control over the RNA release profile may be further overcome by the incorporation of cationic polymers.
RNA nanocarriers
The delivery of RNA nanocarriers (for example, liposomes/lipid NPs, polymeric NPs and inorganic nanomaterials) loaded within a hydrogel network could avoid the chemical modification of both RNA and hydrogel polymers and improve loading, stability and transfection efficiency compared with naked RNA strategies (Fig. 3)3. Below, we outline the loading of several representative RNA nanocarriers in hydrogels.
Lipid nanocarrier
Cationic/ionizable lipids can be used in the form of either liposomes or RNA/lipid complexes, such as lipoplexes and niosomes57. Fibrin gels are capable of supporting cell migration while maintaining lentivirus activity58. Fibrin-based hydrogel surface was conjugated with siRNA-loaded lipofectamine to increase its cellular internalization levels and knock down antagonists (for example, noggin)59. Approximately 20% of free siRNA or siRNA complexed with lipofectamine was remained on the fibrin surface after 3 days, therefore indicating that the negative charge of fibrin does not appear to influence the surface retention of nanocomplexes. On the other hand, the use of injectable chitosan–alginate scaffolds containing mRNA–lipoplexes demonstrated the ability to induce an in vivo local transfection and increase antibody production and T-cell proliferation levels when compared with the systemic administration of only mRNA–lipoplexes60. Notably, there is still a limited number of published studies using RNA–liposome-loaded hydrogels, which might be explained by the thermodynamic instability of liposomes and their further aggregation in a charged hydrogel61.
Polymer nanocarrier
Cationic polymers such as polyethylenimine (PEI) chitosan, and poly(l-lysine) (PLL) are often used to create polyplexes62. Unlike lipid-based nanocarriers, cationic polymers are completely soluble in water due to their general absence of hydrophobic moieties46. Moreover, cationic polymers have the ability to compress nucleic acids to a smaller size than cationic lipids. One potential disadvantage of RNA–polymer nanocarriers is that, for some soft and charged NPs, aggregation could occur during loading, which may limit the amount of RNA that can be loaded into the hydrogel63. Collagen-based hydrogels, which closely resemble natural ECM, have been used for local delivery of RNA nanocomplexes. One specific collagen hydrogel was capable of sustained delivery of siRNA/PEI nanocomplexes in vitro over 10 days32. However, the foreign proteins in collagen hydrogels might elicit a foreign body response, which could hinder their biological application64. Along these lines, HA-based hydrogels may be one of the preferred choices for RNA delivery65. Cyclooxygenase-engineered miRNA (COX-1 and COX-2) plasmids were loaded onto PLGA/PEI NP complexes, and then embedded in HA hydrogels65. A slower, more sustained release profile from the hydrogel was detected compared with plasmid/NP complexes. Possible interactions of polymeric nanocarriers with the hydrogel could affect the release rate. In fact, aggregation and deactivation of RNA-loaded NPs within hydrogels have been reported63. To address this, coating NPs with agarose66, covalent attachment of NPs to the hydrogel backbone67 or similar strategies may be applied in engineering such delivery systems.
Inorganic nanocarrier
Inorganic colloidal NPs (for example, gold, iron oxide, silica and quantum dots) have been utilized in RNA therapy mainly for their facile synthesis process and wide availability3. For example, PEI-based hydrogels were conjugated with siRNA-Au-Fe3O4 nanocapsule. The subcutaneous administration of nanocapsule hydrogel demonstrated a better tumour penetration and a higher blood circulation time compared with intravenous administration of nanocapsule only22. Certainly, AuNPs allow a wide variety of functionalizations via gold–thiol conjugation, being broadly used in multi-modal approaches68. Multifunctional quantum dot DNA hydrogels containing doxorubicin and siRNA reduced EGFR expression significantly more than siRNA alone69. Quantum dot DNA hydrogels are capable of functioning as a delivery vector without toxic transfection agents and have demonstrated high in vivo therapeutic efficacy against breast cancer. The combination of inorganic core–hydrogel scaffolds with colloidal NPs introduces the possibility of new scaffold structures with different core size, charge, coatings and physical stretching/compression of hydrogels.
Hydrogel design for controlled RNA release
The profile release of RNA from the engineered hydrogel network, including the duration of RNA availability (short term versus long term) and the release pattern (continuous versus pulsatile), will strongly depend on the target application. As a result, multiple hydrogel designs have been employed to facilitate the controlled release of RNAs through either passive or active mechanisms. While passive mechanisms can enable continuous short- and long-term release, active mechanisms can yield pulsatile release patterns. In the following section, a comprehensive review of these mechanisms for controlled release of naked RNAs or RNA-nanocarriers from hydrogels is provided (Fig. 5).
a, The ultimate release profile of the encapsulated naked RNA and/or RNA nanocarriers is determined by the hydrogel’s physical features and RNA–hydrogel interactions. b, Upon local administration, the release of the encapsulated RNA can be triggered by external or internal stimuli. c, Illustrative release profiles of encapsulated naked RNA and/or RNA nanocarriers. d, Illustrative biodistribution profiles of RNA therapeutics administered in the naked form or in combination with a hydrogel system. e, Illustrative local accumulation profiles of payload at the implantation site in the naked form or in combination with a hydrogel system.
Hydrogel for continuous RNA release
Continuous passive release from hydrogels is achieved by the individual action or the combination of factors like diffusion, hydrogel network degradation, and hydrogel swelling. On this account, passive release can be adjusted by engineering hydrogel features such as molecular weight, matrix concentration, crosslinking density, hydrophilicity and pore size distribution33,70,71, in addition to the aforementioned hydrogel chemistries for interacting with RNAs.
Hydrolytically degradable functionalities, such as ester groups, have been incorporated into the hydrogel backbone as a means to control release rate72. To this end, based on thiol–ene interactions, an hydrogel formed in situ was made using a combination of eight-arm thiol-modified PEG (8-arm-PEG-SH) with either eight-arm acrylic-modified PEG (8-arm-PEG-A) that contains one ester group on each arm or eight-arm mono(2-acryloyloxyethyl) succinate-modified PEG (8-arm-PEG-MAES) that contains three hydrolysable ester groups on each arm73. The hydrogel with the higher ester linkages in the macromolecular networks (8-arm-PEG-MAES) exhibited faster swelling and degradation, which induces a faster release of RNA-PEI nanocomplexes (85.09 ± 2.43% over 19 days) compared with the other two hydrogel formulations.
The release rate can be further tuned by adjusting the nanocarrier size and concentration28. Photocrosslinked DEX hydrogels (DEX-HEMA) were covalently functionalized with cationic linear PEI methacrylate (LPEI-GMA) via a biodegradable ester linkage74. siRNA electrostatically interacts with the cationic linear PEI. Thus, siRNA profile release was fine-tuned by the degradation rate of DEX-HEMA hydrogels via biodegradable ester linkages and the degree of siRNA/PEI interactions, which were achieved by controlling the hydrogel (8 and 12 wt%) and/or the nanocarrier (0, 5 and 10 μg) concentration. The hydrogels were able to release the siRNA over long time periods (9 to 17 days). Remarkably, the observed sustained profile release of RNA from hydrogel network over a long time period (weeks) can be detrimental to induce a significant physiological response. Indeed, the low amount of RNA that is released from the hydrogel network cannot be sufficient to promote a target effect that systemic administration (or several local administrations) can achieve.
Hydrogels for stimuli-responsive RNA release
To address the issues associated with passive release, on-demand delivery of RNAs from the hydrogels has been achieved using active mechanisms of release. Active release is achieved in response to internal (for example, pH and enzyme) or external stimuli (for example, photoradiation), which can facilitate on-demand degradation of the hydrogel network and hence promote RNA release. The trigger of RNA release through external stimuli introduces additional control of RNA delivery at defined doses and during specific periods, providing an alternative to physician- or patient-delivered strategies.
pH-responsive release
pH-responsive hydrogels often contain Schiff base bonds, which are stable at pH 7.4 but disrupted in an acidic environment (for example, pH 6.8)75. Such hydrogels are suitable for on-demand release of RNA in diseases associated with an acidic tissue microenvironment (such as cancer and myocardial infarction (MI)). For instance, a pH-sensitive hydrogel was created based on a combination of miRNA-loaded amine-functionalized mesoporous silica NPs (MSNs), aldehyde-functionalized PEG (PEGCHO) and α-CD76. The hydrogel fabrication relies on Schiff and hydrophobic interactions between PEGCHO, α-CD and MSN. At a slightly acidic environment (pH 6.8), Schiff base bonds are cleaved to produce an aldehyde functional group, and MSN/miR-21-5p is released from the hydrogel (75% for 1 week in vitro) to the infarct region. While at pH 7.4, only 6% of MSN/siRNA nanocomplexes were released from the hydrogel. Interestingly, the amount of released RNA upon pH stimuli was effective as an MI treatment.
Enzyme-responsive release
Enzyme-sensitive hydrogels normally contain a polymeric network that is crosslinked with an enzyme-sensitive peptide linker77. In the presence of a certain enzyme (for example, matrix metallopeptidase 2 (MMP-2), protease, trypsin and lysozyme) the peptide linker is broken, which leads to the release of the entrapped RNA therapeutics from the hydrogels. Along these lines, HA-based hydrogels were formed through hydrozone bonds (that is, aldehyde-modified HA and hydrazide-modified HA) and protease degradable peptide crosslinkers78. Then, CD-modified HA was introduced in the hydrogel system to sequester cholesterol-modified siRNA, as previously described40. As expected, the hydrogel was eroded and siRNA targeting MMP2 was released in response to protease (for example, collagenase) levels for MI treatment60. In another study, an MMP-2 degradable hydrogel was loaded with tumour growth factor-β1 siRNA polyplexes, which were further adsorbed onto electrospun fibres79. High MMP-2 concentrations promoted faster release of polyplexes due to the MMP-2 substrate peptide degradation in the hydrogels, whereas the addition of MMP-2 had almost no influence on siRNA release from the hydrogels containing MMP-2-nondegradable crosslinkers.
Photo-triggered release
Light-responsive hydrogels offer on-demand spatial and temporal control. Generally, these hydrogels contain single or multiple photocleavable moieties (for example, nitrobenzyl-based linkers with ester or amide bonds) with variable degradation properties in response to light wavelength and intensity, as well as radiation time. While naked RNA is attached to the hydrogel network via photolabile bonds, the RNA nanocarriers are encapsulated in the hydrogel crosslinked by these photo-sensitive bonds. Photodegradable PEG-di(photolabile acrylate) (PEG-DPA) has been consistently used as a building block in these hydrogels50. Upon ultraviolet (UV) light exposure, ester groups linked to ortho-nitrobenzyl photolabile groups cleave into acetal and acidic moieties, promoting siRNA release. Photolabile hydrogels have been also prepared using Michael addition to control the release of siRNA-PEI nanocomplexes80. The photodegradable hydrogels with the lowest amount of photolabile moieties showed an increase in the hydrolytic degradation rate of ester bonds, which enhances siRNA therapeutics release. In addition, the siRNA profile release from photolabile hydrogels was also affected by UV light exposure. Remarkably, the selective delivery of miRNA was achieved by using PEG-based hydrogels via a copper-free click reaction and conjugated with UV-cleavable Chol-miR-26a, which allows control over miRNA release by tailoring UV irradiation time and UV intensity.
Altogether, proper design of hydrogels with photo-degradable linkers can achieve on-demand release of RNA therapeutics upon UV radiation. However, due to the low penetration of UV light, these hydrogels may not be suitable for use in deep tissues.
Biomedical applications
Hydrogel delivery of RNA therapeutics has been applied in diverse biomedical applications (Table 2). Hydrogels are primarily used to facilitate local administration of RNA therapeutics to the disease site and to protect the RNA from innate immune responses. However, depending on the disease pathology, hydrogels can be modified to yield various release profiles to maximize the efficacy of RNA therapeutics. Below, we primarily highlight the use of RNA delivery hydrogels in cancer therapy, bone regeneration, cardiac repair and wound healing (Fig. 6).
Cancer therapy
Systemic cancer treatment is useful for metastasis but is also associated with systemic toxicity and possible immunogenicity due to leakage/accumulation in major organs. In this context, hydrogels could facilitate long-term and sustained local delivery of RNA therapeutics to reduce potential side effects while attacking the primary tumour24,67, reprogramming the primary tumour to prevent metastasis81 and/or inhibiting the recurrence of the primary tumour after surgical resection68. Accordingly, different types of activated oncogene mRNA or miRNA can be effectively inhibited by RNAi technology to inhibit tumour growth. For example, NPs containing RNA therapeutics (for example, miRNA) can be embedded in a hydrogel matrix, which is in turn implanted next to the tumour24,81. Remarkably, a hydrogel network composed of a two-component system, namely Schiff-base interactions between an oxidized polysaccharide and an amine-containing dendrimer, demonstrated the use of the ratio of aldehyde to amine groups to control the release rate of RNA therapeutics. However, highly crosslinked hydrogels can hinder the release of embedded RNA therapeutics, reducing therapeutic efficacy. To address this issue, one-component injectable polyplex hydrogels were used to deliver RNA therapeutics (for example, siRNA) with higher efficiency19,21. Typically, these polyplex systems contain a thermo-sensitive moiety that allows sol-to-gel transition upon injection into the target tissue. Subsequently, the release rate of RNA-containing polyplexes from these gels depends on their dissolution rate, which can be further controlled by incorporating degradable linkers sensitive to hydrolysis or enzymatic activity. Consequently, hydrogel-mediated delivery of RNA therapeutics for cancer therapy entails prolonged retention of the nano-vectors around the tumour, which enhances uptake by the particular cancer cell population. This becomes crucial when dealing with brain tumours, as such platforms are capable of overcoming biological barriers (mainly the blood–brain barrier) to deliver therapeutics to the brain tissue. In general, hydrogels for the delivery of RNA therapeutics in cancer have demonstrated enhanced bioavailability and increased tumour accumulation with less homing in non-target tissues. In the context of cancer, RNA hydrogel delivery is not limited to silencing of oncogenic genes, and the next great achievement on the horizon could be manipulation of immunomodulatory factors to recruit immune cells for cancer immunotherapy82.
Bone regeneration
Bone regeneration and repair is another arena in which hydrogel-mediated RNA delivery has shown promise72,75. Bone healing relies on several dynamic and spatiotemporal mechanisms, including inflammatory, repair, and remodeling phases at crucial cellular (that is, inflammatory cells, vascular cells, osteochondral progenitors, and osteoclasts) and molecular levels (that is, pro-inflammatory cytokines, growth factors, and angiogenic and pro-osteogenic factors)83. As a result, it is crucial for the hydrogels to modulate the spatio-temporal and dose-controlled release of RNA therapeutics to meet the highly complex microenvironment present during bone regeneration. Correspondingly, if the hydrogels are to be implemented as a scaffold, their degradation should correlate with the rate of ingrowing tissue to provide sufficient mechanical support. Given such requirements, photodegradable hydrogels have shown immense potential to facilitate the on-demand release of RNA therapeutics80. In these systems, the hydrogels contain both hydrolytically degradable linkages (for example, disulfide and/or ester bonds) as well as photolytically degradable sites (thiol-acrylate bonds). Therefore, UV radiation induces photodegradation of photolabile linkages in the hydrogel network, affecting the hydrogel’s physiochemical properties such as swelling and degradation rate. The results showed that UV radiation can lead to faster release of siRNA from these hydrogels, and that the associated release rate can be further modified by adjusting the ratio of photolabile groups in the hydrogel composition. Hydrogels of this sort allow temporal tuning of RNAi presentation directly at the diseased site, which is known to enhance bone formation84. Although research studies have explored the delivery of osteogenesis-inducing therapeutic agents, it is also possible to study additional cell responses, including angiogenesis and cell infiltration.
Cardiac repair
MI results from coronary artery occlusion, producing local ischemia, tissue damage, and ultimately, heart failure. There are interesting approaches that have been applied to enhance ECM homeostasis and angiogenesis or to prevent fibrosis and calcium imbalance, namely the delivery of siRNA, miRNA and short hairpin RNA (shRNA)85. Remarkably, the use of self-healing hydrogels that are injected into the infarct site using minimally invasive approaches (for example, catheters) and are able to reform after this shear cessation has been a promising option to the challenge of delivery and retention of RNAi therapeutics86. To this end, a variety of chemistries, including ionic bonds87 and dynamic covalent bonds (hydrazone linkages25 and guest–host interactions40), have been implemented to yield injectable self-healing hydrogels for RNAi delivery. Among them, guest–host hydrogels (involving CD molecules) can facilitate a slower release of cholesterol-modified RNAi via hydrophobic interactions. Stimuli-responsive linkages (for example, protease-sensitive78 and pH-responsive76) were also utilized as other means to achieve on-demand release from injectable self-healing hydrogels. Particularly, these two stimuli were chosen because MI is associated with changes in the tissue microenvironment, including a reduction in pH (from 7.4 to 6.8) and local upregulation of proteolytic activity. This emphasizes the importance of considering disease pathology when designing hydrogels for RNA delivery. In general, a significant advantage of RNAi delivery using injectable self-healing hydrogels is that single-dose administration of such systems can significantly and continuously restore the infarcted myocardium and enhance cardiac function over an extended period (from one to three months). Given the role of the immune response in MI disease progression and repair, hydrogels can play an essential role in delivering RNA therapeutics for manipulation of macrophages and regulatory T cells, limiting pro-inflammatory responses and increasing regenerative cytokines in the infarct region.
Wound healing
Wound healing is a well orchestrated and regulated process that can be divided into three overlapping phases: haemostasis and inflammation, proliferation, and tissue remodeling88. However, this process becomes severely dysregulated by pathophysiological conditions. RNAi therapeutics have already shown potential in addressing all three phases and therefore facilitating functional tissue regeneration. Hydrogels can play a central role in both the local delivery of RNAi therapeutics and providing an artificial matrix to aid in the healing process. For instance, thermo-responsive hydrogels (for example, pluronic F-127, methylcellulose and agarose) have commonly been used to deliver miRNA or siRNA to accelerate wound healing89. One disadvantage of such hydrogels may be the inability to control the release rate of the encapsulated RNAi therapeutics.
To address this issue, both physically and chemically crosslinked hydrogels have been employed, and the degree of crosslinking was used to control the release rate of RNAi therapeutics. A common example of physically crosslinked hydrogels is the layer-by-layer assembly of two oppositely charged biopolymers90. Increasing the number of layers led to a slow release of RNAi therapeutics from these hydrogels. Given their physical nature, these gels are capable of releasing RNAi over a span of 2 weeks. For chemically crosslinked hydrogels, other variables can also affect the release rate of RNAi therapeutics. This generally depends on the entities involved in forming the chemical crosslinks within the hydrogel. For instance, the crosslinking could be a result of Schiff base bonds between aldehyde and amine groups on polymer matrixes91. These hydrogels often degrade slowly over a period of a month, and hence they yield a longer release profile for RNAi therapeutics. Conversely, hydrogels formed as a result of interactions between the polymer matrix and the RNAi nano-vector are degraded more quickly (maximum, seven days), yielding a shorter release profile92,93. In these cases, there is a direct correlation between the number of active functional groups on the nano-vector and the corresponding hydrogel crosslinking density. As a result, the release rate of encapsulated RNAi can be simply tuned by adjusting the concentration of nanocarrier in the hydrogel. Notably, nanocarriers with active surface functionalities can be incorporated into chemically crosslinked hydrogel networks as a way to provide more control over the release rate of RNAi therapeutics28. The miRNA-laden hydrogels drive wound healing by triggering resolution of the inflammatory phase. The results showed elevated macrophage infiltration and effective local polarization of macrophages toward the M2 phenotype in vivo. Hydrogels, with their remarkably high water uptake, support cell attachment and growth, leading to better wound healing. These hydrogel examples yield a spectrum of different temporal release profiles for RNAi therapeutics, which is particularly beneficial when considering that wound healing consists of a carefully orchestrated sequence of biological events.
Notably, the risk of infection or localized trauma is commonly associated with the use of conventional methods for wound closure (for example, sutures). Therefore, the use of hydrogel-based adhesives, which strongly attach to the wound, can be used as a physical barrier to protect the wound from the above-mentioned risks that will strongly affect re-epithelialization and healing rate94.
Other applications
RNA molecules have received a great deal of attention for immunomodulation, mainly due to RNA silencing of crucial factors in immune cells95. Adhesive hydrogels have been used as immunomodulatory dressings. For the combined delivery of dendritic cell (DC) chemo-attractants (MIP3a) and pDNA-siRNA-loaded microparticles to antigen-presenting cells, an in situ crosslinkable and fast-degrading hydrogel was developed96. DCs were able to infiltrate the hydrogels and efficiently phagocytose the microparticles carrying pDNA-siRNA. The gels attracted four- to sixfold more DCs compared with an equivalent bolus dose. A more recent study showed that, upon the in vivo delivery of mRNA–lipoplexes loaded into chitosan–alginate hydrogel, T-cell proliferation and interferon-γ secretion were both increased60. At week 1, a humoral response was observed for lipoplex-loaded hydrogels, while protein-based vaccines did not elicit IgG production until 2 weeks post-injection, which potentiated their applications as a viable immunization method against multiple diseases.
The process of angiogenesis is closely tied to wound healing and tissue regeneration. Stimulating both the formation and maturation of blood vessels is therefore of great interest, especially in the treatment of some chronic skin wounds. Localized silencing of ubiquitously expressed genes (for example, mapk-1) in an open wound bed was demonstrated using an agarose hydrogel loaded with liposomal siRNA97. Delivery of siRNA NPs via hydrogels incorporating polyurethane (PUR) and its derivatives, polyester urethane (PEUR) or poly(thioketal urethane) (PTK-UR), was also explored for angiogenesis98. Modulating the release rate caused changes in the in vivo silencing profile. Silencing prolyl hydroxylase domain protein 2 (PHD2) resulted in the expression of vascular endothelial growth factor and fibroblast growth factor, while vascular volume and thickness within the hydrogels also increased. The use of these hydrogels for local PHD2 siRNA delivery showed excellent promise for promoting angiogenesis for wound healing.
Some other reported applications, such as spinal cord injury, fibrosis and inflammatory diseases, are summarized in Table 2.
Summary and future directions
Macroscale delivery systems that can be locally implanted on the disease tissue while avoiding the complications associated to the systemic delivery of RNA therapeutics, have captured the attention of researchers in the field in the past decades. Particularly, hydrogels can be used to efficiently deliver both small and macro molecules, like chemotherapeutics, proteins and genetic materials (such as RNA), along with nanoparticle-based therapies. Hydrogels as a three-dimensional matrix framework have gained attention in therapeutics due to their biocompatibility, biodegradability, drug loading ability and controlled drug release. Compared with systemic administration, hydrogel systems have many advantages, such as locally controlled RNA delivery, low blood RNA concentration, high permeability, few toxic side effects, avoidance of first-pass hepatic metabolism, and minimal pain and discomfort70,75. The combination of a local platform to treat and re-educate the disease tissue along with systemic administration to treat an existing distant disease niche would impart highly efficacious translational therapeutic platforms with improved clinical outcomes81. To take this to the next level, macroscale hydrogels have recently been developed with nano/micro hydrogel building blocks99. Hydrolytic degradation of macroscale hydrogels then allowed for the gradual release of RNA-loaded nano/micro hydrogels without leaving any residual biomaterial at the disease site after treatment. The balance between hydrogel-RNA design complexity, manufacturing costs, regulatory policies and the effective release into the target tissue needs to be evaluated in detail so these strategies can be commonly applied in clinical procedures.
Disease location and targeted tissue will dictate the necessary physical attributes of the hydrogel while disease type will determine the suitable spatiotemporal RNA release profile. Injectable hydrogels with self-healing properties are extremely useful for heart delivery, while topical hydrogels with adhesive properties are preferred for RNA delivery to the skin. For instance, self-healing hydrogels can withstand the shear forces during injection as well as the dynamic forces generated by the beating muscles after myocardium injection. Further parameters must be taken into consideration when hydrogels are also intended to serve as a scaffolding matrix to promote tissue regeneration. This includes parameters such as mechanical robustness and degradation rate of the hydrogels, which can inevitably affect the release rate of encapsulated RNA therapeutics. For example, RNA delivery to injured bone tissue must be commensurate with the healing timeline (approximately 3–4 weeks)33. Hence, depending on the type of RNA and its association with a specific healing phase, the delivery timeline could vary from several days to several months100. Additionally, disease-associated changes to a tissue microenvironment, such as altered pH or upregulation of certain enzymes, can be implemented in the hydrogel design to trigger RNA release.
As mentioned above, biomedical applications of hydrogel-mediated RNA delivery can range from tissue regeneration to cancer therapy. However, one unexplored area is the employment of hydrogel scaffolds to support RNA–cell interactions. Here, hydrogels function as a staging area for gene regulation and engineering as cells migrate into the hydrogels for interaction with RNAs. This concept has been utilized in incorporating previously gene-modified human mesenchymal stromal cells into a cryogel scaffold101. These genetically modified cells can release certain antibodies capable of triggering T-cell-mediated anti-tumour responses. Ultimately, hydrogel scaffolds for RNA-cell interactions could have a combinatorial effect by simultaneously editing certain genes in the cells and supporting their proliferation and survival, ensuring the constant release of effective levels of antibodies.
Another promising application of hydrogels in this field is the delivery of RNA nano-vaccines. The coronavirus disease 2019 (COVID-19) pandemic is still raging all over the world, and vaccination is the best defence. After unremitting efforts, two mRNA vaccines based on lipid NPs (BNT162b2 by Pfizer/BioNTech and mRNA-1273 by Moderna) are now in clinical use. Injectable hydrogels were recently used for local delivery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymeric nano-vaccines (containing the SARS-CoV-2 virus spike protein with/without adjuvant) in animal models102. Interestingly, the results show that sustained delivery of the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein nano-vaccine in an injectable hydrogel depot formulation achieved higher total anti-RBD IgG titres compared to bolus vaccine controls. A similar concept can be applied to current SARS-CoV-2 mRNA vaccines by incorporating them into hydrogels. Currently, these mRNA nano-vaccines require two doses separated by 3-4 weeks. The use of hydrogel capsules with pulsatile release could release the vaccines in pulses over weeks 3–4, which if injected along with free nano-vaccines, might provide one-shot vaccination103. However, it should also be noted that little has been done to examine the in vivo stability of mRNA in hydrogels at physiological conditions for such a long period of time, which will be an important area for future studies. Indeed, for larger RNAs such as mRNA, suboptimal stability elicits only short-term transient protein expression and requires delivery vehicles like NPs to protect them from enzymatic degradation and improve their transfection efficiency. The use of hydrogels for naked mRNA delivery has rarely been reported. Thus, for future efforts to modify large RNAs to improve stability and transfection, the application of hydrogels for naked RNA delivery will be more fruitful. Another issue with mRNA nano-vaccines is that they must be stored and shipped at low temperatures. Given the current scant evidence, identifying hydrogels suitable for long-term mRNA storage at 4 °C or even room temperature will be important. With further research, hydrogel-mediated mRNA vaccine delivery may become a viable alternative to traditional nucleic acid immunization methods.
In this Review, we have discussed the use of hydrogels as RNA delivery systems from design to biomedical applications. The research discussed herein demonstrates that hydrogel systems are capable not only of sustained local delivery of RNA (which avoids repeated administration) but also of spatial and temporal control over release rate. Further investigation in vivo of the characteristics of RNA-loaded hydrogels, such as degradability, clearance, controlled release and foreign body response, is urgently needed. It is expected that continuous improvements in hydrogel design and fabrication will bring these exciting materials ever closer to clinical applications of RNA therapy.
References
Yin, H. et al. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 15, 541–555 (2014).
Wu, S. Y., Lopez-Berestein, G., Calin, G. A. & Sood, A. K. RNAi therapies: drugging the undruggable. Sci. Transl. Med. 6, 240ps7 (2014).
Mendes, B. B. et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Primers 2, 24 (2022).
Sahin, U., Karikó, K. & Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).
Davidson, B. L. & McCray, P. B. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 12, 329–340 (2011).
Peng, Y. & Croce, C. M. The role of microRNAs in human cancer. Signal Transduct. Target Ther. 1, 15004 (2016).
Paunovska, K., Loughrey, D. & Dahlman, J. E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 23, 265–280 (2022).
Ku, S. H., Jo, S. D., Lee, Y. K., Kim, K. & Kim, S. H. Chemical and structural modifications of RNAi therapeutics. Adv. Drug Deliv. Rev. 104, 16–28 (2016).
Lee, S. H., Kang, Y. Y., Jang, H.-E. & Mok, H. Current preclinical small interfering RNA (siRNA)-based conjugate systems for RNA therapeutics. Adv. Drug Deliv. Rev. 104, 78–92 (2016).
Kulkarni, J. A., Witzigmann, D., Chen, S., Cullis, P. R. & van der Meel, R. Lipid nanoparticle technology for clinical translation of siRNA therapeutics. Acc. Chem. Res. 52, 2435–2444 (2019).
Bergen, J. M., Park, I.-K., Horner, P. J. & Pun, S. H. Nonviral approaches for neuronal delivery of nucleic acids. Pharm. Res. 25, 983–998 (2008).
Lokugamage, M. P., Sago, C. D., Gan, Z., Krupczak, B. R. & Dahlman, J. E. Constrained nanoparticles deliver siRNA and sgRNA to T cells in vivo without targeting ligands. Adv. Mater. 31, 1902251 (2019).
Fenton, O. S., Olafson, K. N., Pillai, P. S., Mitchell, M. J. & Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 30, 1705328 (2018).
Matz, R. L. et al. Polyplex exposure inhibits cell cycle, increases inflammatory response, and can cause protein expression without cell division. Mol. Pharm. 10, 1306–1317 (2013).
Singh, A. et al. An injectable synthetic immune-priming center mediates efficient T-cell class switching and T-helper 1 response against B cell lymphoma. J. Control. Release 155, 184–192 (2011).
Manaka, T. et al. Local delivery of siRNA using a biodegradable polymer application to enhance BMP-induced bone formation. Biomaterials 32, 9642–9648 (2011).
Kozlowska, A. K. et al. Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment. Acta Biomater. 59, 221–233 (2017).
Takahashi, H., Wang, Y. & Grainger, D. W. Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation. J. Control. Release 147, 400–407 (2010).
Kim, Y.-M., Park, M.-R. & Song, S.-C. Injectable polyplex hydrogel for localized and long-term delivery of siRNA. ACS Nano 6, 5757–5766 (2012).
Browne, S. et al. Modulation of inflammation and angiogenesis and changes in ECM GAG-activity via dual delivery of nucleic acids. Biomaterials 69, 133–147 (2015).
Kim, Y.-M. & Song, S.-C. Targetable micelleplex hydrogel for long-term, effective, and systemic siRNA delivery. Biomaterials 35, 7970–7977 (2014).
Zhang, Z.-Q., Kim, Y.-M. & Song, S.-C. Injectable and quadruple-functional hydrogel as an alternative to intravenous delivery for enhanced tumor targeting. ACS Appl. Mater. Interfaces 11, 34634–34644 (2019).
Bhosle, S. M. et al. Unifying in vitro and in vivo IVT mRNA expression discrepancies in skeletal muscle via mechanotransduction. Biomaterials 159, 189–203 (2018).
Conde, J., Oliva, N., Atilano, M., Song, H. S. & Artzi, N. Self-assembled RNA-triple-helix hydrogel scaffold for microRNA modulation in the tumour microenvironment. Nat. Mater. 15, 353–363 (2016).
Yang, H. et al. An in vivo miRNA delivery system for restoring infarcted myocardium. ACS Nano 13, 9880–9894 (2019).
Han, C. et al. Delivery of miR-675 by stem cell-derived exosomes encapsulated in silk fibroin hydrogel prevents aging-induced vascular dysfunction in mouse hindlimb. Mater. Sci. Eng. C 99, 322–332 (2019).
Lu, Y.-J. et al. Injectable thermo-sensitive chitosan hydrogel containing CPT-11-loaded EGFR-targeted graphene oxide and SLP2 shRNA for localized drug/gene delivery in glioblastoma therapy. Int. J. Mol. Sci. 21, 7111 (2020).
Saleh, B. et al. Local immunomodulation using an adhesive hydrogel loaded with miRNA-laden nanoparticles promotes wound healing. Small 15, 1902232 (2019).
Lin, Q. et al. Injectable supramolecular hydrogel formed from α-cyclodextrin and PEGylated arginine-functionalized poly(l-lysine) dendron for sustained MMP-9 shRNA plasmid delivery. Acta Biomater. 49, 456–471 (2017).
Alam, P. et al. Inhibition of senescence‐associated genes Rb1 and Meis2 in adult cardiomyocytes results in cell cycle reentry and cardiac repair post–myocardial infarction. J. Am. Heart Assoc. 8, e012089 (2019).
San Juan, A. et al. Development of a functionalized polymer for stent coating in the arterial delivery of small interfering RNA. Biomacromolecules 10, 3074–3080 (2009).
Peng, H. et al. Sustained delivery of siRNA/PEI complex from in situ forming hydrogels potently inhibits the proliferation of gastric cancer. J. Exp. Clin. Cancer Res. 35, 57 (2016).
Wang, Y., Malcolm, D. W. & Benoit, D. S. W. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 139, 127–138 (2017).
Ding, F. et al. A crosslinked nucleic acid nanogel for effective siRNA delivery and antitumor therapy. Angew. Chem. Int. Ed. 57, 3064–3068 (2018).
Ma, H. et al. PLK1shRNA and doxorubicin co-loaded thermosensitive PLGA-PEG-PLGA hydrogels for osteosarcoma treatment. Biomaterials 35, 8723–8734 (2014).
Miura, N. et al. Human RGM249-derived small RNAs potentially regulate tumor malignancy. Nucleic Acid Ther. 23, 332–343 (2013).
Chun, Y. Y. et al. Positive-charge tuned gelatin hydrogel-siSPARC injectable for siRNA anti-scarring therapy in post glaucoma filtration surgery. Sci. Rep. 11, 1470 (2021).
Radmanesh, F. et al. Hydrogel-mediated delivery of microRNA-92a inhibitor polyplex nanoparticles induces localized angiogenesis. Angiogenesis 24, 657–676 (2021).
Li, Y. et al. The promotion of bone regeneration through positive regulation of angiogenic–osteogenic coupling using microRNA-26a. Biomaterials 34, 5048–5058 (2013).
Wang, L. L. et al. Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nat. Biomed. Eng. 1, 983–992 (2017).
Prince, E. & Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 4, 99–115 (2019).
Li, Y., Xiao, Y. & Liu, C. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 117, 4376–4421 (2017).
Vázquez-González, M. & Willner, I. Stimuli-responsive biomolecule-based hydrogels and their applications. Angew. Chem. Int. Ed. 59, 15342–15377 (2020).
Wang, L. L. et al. Injectable, guest–host assembled polyethylenimine hydrogel for siRNA delivery. Biomacromolecules 18, 77–86 (2017).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976).
Lv, H., Zhang, S., Wang, B., Cui, S. & Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 114, 100–109 (2006).
Krebs, M. D., Jeon, O. & Alsberg, E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J. Am. Chem. Soc. 131, 9204–9206 (2009).
Talebian, S. et al. Self-healing hydrogels: the next paradigm shift in tissue engineering? Adv. Sci. 6, 1801664 (2019).
Paidikondala, M., Nawale, G. N. & Varghese, O. P. Insights into siRNA transfection in suspension: efficient gene silencing in human mesenchymal stem cells encapsulated in hyaluronic acid hydrogel. Biomacromolecules 20, 1317–1324 (2019).
Huynh, C. T. et al. Photocleavable hydrogels for light-triggered siRNA release. Adv. Health. Mater. 5, 305–310 (2016).
Nguyen, M. K. et al. Covalently tethering siRNA to hydrogels for localized, controlled release and gene silencing. Sci. Adv. 5, eaax0801 (2019).
Sánchez-Iglesias, A. et al. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano 6, 11059–11065 (2012).
Xu, C., Wu, Y.-L., Li, Z. & Loh, X. J. Cyclodextrin-based sustained gene release systems: a supramolecular solution towards clinical applications. Mater. Chem. Front. 3, 181–192 (2019).
Bakker, M. H., van Rooij, E. & Dankers, P. Y. W. Controlled release of RNAi molecules by tunable supramolecular hydrogel carriers. Chem. Asian J. 13, 3501–3508 (2018).
Li, S.-Y. et al. Restoring anti-tumor functions of T cells via nanoparticle-mediated immune checkpoint modulation. J. Control. Release 231, 17–28 (2016).
Yang, H. et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 23, 214–228 (2015).
Grijalvo, S., Mayr, J., Eritja, R. & Díaz, D. D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: a marriage of convenience. Biomater. Sci. 4, 555–574 (2016).
Kidd, M. E., Shin, S. & Shea, L. D. Fibrin hydrogels for lentiviral gene delivery in vitro and in vivo. J. Control. Release 157, 80–85 (2012).
Kowalczewski, C. J. & Saul, J. M. Surface-mediated delivery of siRNA from fibrin hydrogels for knockdown of the BMP-2 binding antagonist noggin. Acta Biomater. 25, 109–120 (2015).
Yan, J., Chen, R., Zhang, H. & Bryers, J. D. Injectable biodegradable chitosan-alginate 3D porous gel scaffold for mRNA vaccine delivery. Macromol. Biosci. 19, e1800242 (2019).
Wang, L. L. & Burdick, J. A. Engineered hydrogels for local and sustained delivery of RNA-interference therapies. Adv. Health. Mater. 6, 1601041 (2017).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Lei, Y. et al. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials 31, 9106–9116 (2010).
Delgado, L. M., Bayon, Y., Pandit, A. & Zeugolis, D. I. To cross-link or not to cross-link? Cross-linking associated foreign body response of collagen-based devices. Tissue Eng. Part B 21, 298–313 (2014).
Zhou, Y. L. et al. Localized delivery of miRNAs targets cyclooxygenases and reduces flexor tendon adhesions. Acta Biomater. 70, 237–248 (2018).
Tokatlian, T., Cam, C., Siegman, S. N., Lei, Y. & Segura, T. Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs. Acta Biomater. 8, 3921–3931 (2012).
Segovia, N. et al. Hydrogel doped with nanoparticles for local sustained release of siRNA in breast cancer. Adv. Health. Mater. 4, 271–280 (2015).
Conde, J., Oliva, N., Zhang, Y. & Artzi, N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 15, 1128–1138 (2016).
Zhang, L. et al. Multifunctional quantum dot DNA hydrogels. Nat. Commun. 8, 381 (2017).
Xu, Y. et al. Extended-release of therapeutic microRNA via a host-guest supramolecular hydrogel to locally alleviate renal interstitial fibrosis. Biomaterials 275, 120902 (2021).
McMillan, A. et al. Hydrogel microspheres for spatiotemporally controlled delivery of RNA and silencing gene expression within scaffold-free tissue engineered constructs. Acta Biomater. 124, 315–326 (2021).
Nguyen, M. K. et al. RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomater. 75, 105–114 (2018).
Nguyen, M. K., Jeon, O., Krebs, M. D., Schapira, D. & Alsberg, E. Sustained localized presentation of RNA interfering molecules from in situ forming hydrogels to guide stem cell osteogenic differentiation. Biomaterials 35, 6278–6286 (2014).
Nguyen, K., Dang, P. N. & Alsberg, E. Functionalized, biodegradable hydrogels for control over sustained and localized siRNA delivery to incorporated and surrounding cells. Acta Biomater. 9, 4487–4495 (2013).
Chen, J. et al. Injectable self-healing hydrogel with siRNA delivery property for sustained STING silencing and enhanced therapy of intervertebral disc degeneration. Bioact. Mater. 9, 29–43 (2021).
Li, Y. et al. Injectable hydrogel with MSNs/microRNA-21-5p delivery enables both immunomodification and enhanced angiogenesis for myocardial infarction therapy in pigs. Sci. Adv. 7, eabd6740 (2021).
Knipe, J. M., Strong, L. E. & Peppas, N. A. Enzyme- and pH-responsive microencapsulated nanogels for oral delivery of siRNA to induce TNF-α knockdown in the intestine. Biomacromolecules 17, 788–797 (2016).
Wang, L. L. et al. Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. J. Control. Release 285, 152–161 (2018).
Cai, C. et al. MMP-2 responsive unidirectional hydrogel-electrospun patch loading TGF-β1 siRNA polyplexes for peritendinous anti-adhesion. Adv. Funct. Mater. 31, 2008364 (2021).
Huynh, C. T. et al. Cytocompatible catalyst-free photodegradable hydrogels for light-mediated RNA release to induce hMSC osteogenesis. ACS Biomater. Sci. Eng. 3, 2011–2023 (2017).
Gilam, A. et al. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat. Commun. 7, 12868 (2016).
Duong, H. T. T. et al. Degradation-regulated architecture of injectable smart hydrogels enhances humoral immune response and potentiates antitumor activity in human lung carcinoma. Biomaterials 230, 119599 (2020).
Tsekoura, E. K., Remant, B. K. C. & Hasan, U. Biomaterials to facilitate delivery of RNA agents in bone regeneration and repair. ACS Biomater. Sci. Eng. 3, 1195–1206 (2017).
Zhang, X., Li, Y., Chen, Y. E., Chen, J. & Ma, P. X. Cell-free 3D scaffold with two-stage delivery of miRNA-26a to regenerate critical-sized bone defects. Nat. Commun. 7, 10376 (2016).
Braunwald, E. The war against heart failure: the Lancet lecture. Lancet 385, 812–824 (2015).
Bheri, S. & Davis, M. E. Nanoparticle–hydrogel system for post-myocardial infarction delivery of microRNA. ACS Nano 13, 9702–9706 (2019).
Pandey, R. et al. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post ischemic injury. Am. J. Transl. Res 9, 3120–3137 (2017).
Chouhan, D., Dey, N., Bhardwaj, N. & Mandal, B. B. Emerging and innovative approaches for wound healing and skin regeneration: current status and advances. Biomaterials 216, 119267 (2019).
Lan, B. et al. Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J. Nanobiotechnol. 19, 130 (2021).
Castleberry, S. A. et al. Self-assembled wound dressings silence MMP-9 and improve diabetic wound healing in vivo. Adv. Mater. 28, 1809–1817 (2016).
Yang, L., Zhang, L., Hu, J., Wang, W. & Liu, X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with miRNA-laden nanoparticles for chronic diabetic wound treatment. Int. J. Biol. Macromol. 166, 166–178 (2021).
Li, N. et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 102, 298–314 (2020).
Monaghan, M., Browne, S., Schenke-Layland, K. & Pandit, A. A collagen-based scaffold delivering exogenous microRNA-29B to modulate extracellular matrix remodeling. Mol. Ther. 22, 786–796 (2014).
Lee, P.-Y., Li, Z. & Huang, L. Thermosensitive hydrogel as a Tgf-β1 gene delivery vehicle enhances diabetic wound healing. Pharm. Res. 20, 1995–2000 (2003).
Conde, J., Arnold, C. E., Tian, F. & Artzi, N. RNAi nanomaterials targeting immune cells as an anti-tumor therapy: the missing link in cancer treatment? Mater. Today 19, 29–43 (2016).
Singh, A., Suri, S. & Roy, K. In-situ crosslinking hydrogels for combinatorial delivery of chemokines and siRNA–DNA carrying microparticles to dendritic cells. Biomaterials 30, 5187–5200 (2009).
Thanik, V. D. et al. Topical matrix-based siRNA silences local gene expression in a murine wound model. Gene Ther. 14, 1305–1308 (2007).
Nelson, C. E. et al. Tunable delivery of siRNA from a biodegradable scaffold to promote angiogenesis in vivo. Adv. Mater. 26, 607–614 (2014).
Chyzy, A., Tomczykowa, M. & Plonska-Brzezinska, M. E. Hydrogels as potential nano-, micro- and macro-scale systems for controlled drug delivery. Materials 13, 188 (2020).
Kolanthai, E. et al. Nanoparticle mediated RNA delivery for wound healing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 14, e1741 (2021).
Aliperta, R. et al. Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy. Sci. Rep. 7, 42855 (2017).
Gale, E. C. et al. Hydrogel-based slow release of a receptor-binding domain subunit vaccine elicits neutralizing antibody responses against SARS-CoV-2. Adv. Mater. 33, e2104362 (2021).
McHugh, K. J. et al. Fabrication of fillable microparticles and other complex 3D microstructures. Science 357, 1138–1142 (2017).
Han, H. D. et al. Chitosan hydrogel for localized gene silencing. Cancer Biol. Ther. 11, 839–845 (2011).
Ma, Z. et al. Chitosan hydrogel as siRNA vector for prolonged gene silencing. J. Nanobiotechnol. 12, 23 (2014).
Carthew, J. et al. In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells. Acta Biomater. 101, 249–261 (2020).
Chen, Z. et al. Targeted delivery of CRISPR/Cas9-mediated cancer gene therapy via liposome-templated hydrogel nanoparticles. Adv. Funct. Mater. 27, 1703036 (2017).
Badieyan, Z. S. et al. Transcript-activated collagen matrix as sustained mRNA delivery system for bone regeneration. J. Control. Release 239, 137–148 (2016).
Ledo, A. M. et al. mRNA-activated matrices encoding transcription factors as primers of cell differentiation in tissue engineering. Biomaterials 247, 120016 (2020).
Martin, J. R. et al. Local delivery of PHD2 siRNA from ROS-degradable scaffolds to promote diabetic wound healing. Adv. Health. Mater. 5, 2751–2757 (2016).
Sun, W. et al. Bone-targeted nanoplatform combining zoledronate and photothermal therapy to treat breast cancer bone metastasis. ACS Nano 13, 7556–7567 (2019).
Nguyen, L. H. et al. Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 7, 42212 (2017).
Chen, W. et al. Gene-hydrogel microenvironment regulates extracellular matrix metabolism balance in nucleus pulposus. Adv. Sci. 7, 1902099 (2020).
Cao, C., Yan, C., Hu, Z. & Zhou, S. Potential application of injectable chitosan hydrogel treated with siRNA in chronic rhinosinusitis therapy. Mol. Med. Rep. 12, 6688–6694 (2015).
Su, Y. et al. Chitosan hydrogel doped with PEG-PLA nanoparticles for the local delivery of miRNA-146a to treat allergic rhinitis. Pharmaceutics 12, 907 (2020).
Kanazawa, T. et al. Intra-articular retention and anti-arthritic effects in collagen-induced arthritis model mice by injectable small interfering RNA containing hydrogel. Biol. Pharm. Bull. 40, 1929–1933 (2017).
Lee, J. W. et al. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch. Otolaryngol. Head. Neck Surg. 136, 714–719 (2010).
Ding, L. et al. LINGO-1 shRNA loaded by pluronic F-127 promotes functional recovery after ventral root avulsion. Tissue Eng. Part A 25, 1381–1395 (2019).
Kanazawa, T. et al. Topical anti-nuclear factor-kappa B small interfering RNA with functional peptides containing sericin-based hydrogel for atopic dermatitis. Pharmaceutics 7, 294–304 (2015).
Acknowledgements
This work was supported by the US National Institutes of Health grants R01CA200900, R01HL156362, R01HL159012 and R01HL162367 (to J.S.), the Lung Cancer Discovery Award from the American Lung Association (to J.S.), the Innovation Discovery Grants award from the Mass General Brigham (to J.S.), the European Research Council Starting Grant (ERC-StG-2019-848325 to J.C. and B.B.M.) and the Fundação para a Ciência e a Tecnologia FCT Grant (PTDC/BTM-MAT/4738/2020 to J.C.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.L. declares the following financial interests: Alnylam Pharmaceuticals, Inc. and Moderna, Inc. For a list of entities with which R.L. is involved, compensated or uncompensated, see the Supplementary Note. J.C. is a co-founder and shareholder of TargTex S.A. - Targeted Therapeutics for Glioblastoma Multiforme. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Tatiana Segura, Millicent Sullivan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Note 1
Financial interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, R., Talebian, S., Mendes, B.B. et al. Hydrogels for RNA delivery. Nat. Mater. 22, 818–831 (2023). https://doi.org/10.1038/s41563-023-01472-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-023-01472-w