Abstract
Biointegrated neuromorphic hardware holds promise for new protocols to record/regulate signalling in biological systems. Making such artificial neural circuits successful requires minimal device/circuit complexity and ion-based operating mechanisms akin to those found in biology. Artificial spiking neurons, based on silicon-based complementary metal-oxide semiconductors or negative differential resistance device circuits, can emulate several neural features but are complicated to fabricate, not biocompatible and lack ion-/chemical-based modulation features. Here we report a biorealistic conductance-based organic electrochemical neuron (c-OECN) using a mixed ion–electron conducting ladder-type polymer with stable ion-tunable antiambipolarity. The latter is used to emulate the activation/inactivation of sodium channels and delayed activation of potassium channels of biological neurons. These c-OECNs can spike at bioplausible frequencies nearing 100 Hz, emulate most critical biological neural features, demonstrate stochastic spiking and enable neurotransmitter-/amino acid-/ion-based spiking modulation, which is then used to stimulate biological nerves in vivo. These combined features are impossible to achieve using previous technologies.
Similar content being viewed by others
Main
Neurons communicate and process information using action potentials or spikes in membrane potentials1. This spike generation and the properties of the neuron are defined by voltage-, ion- and neurotransmitter-dependent conductance of various ion channels in the cell membrane. On receiving an electrical input, the cumulative effect of ionic currents through these channels—primarily the sodium and potassium channels—perturbs the membrane voltage from its resting value, resulting in an action potential. Thus, to create a realistic electrical circuit analogue to the biological neuron, one should emulate the conductance of the sodium channel, which shows a fast activation and inactivation, and the potassium channel, which activates after a certain delay. Simple leaky integrate-and-fire model neurons, based on silicon2 or organic semiconductors3,4, do not incorporate such complex ion channel dynamics and hence can emulate only limited neural features. More biorealistic conductance-based neuron models4,5 can be realized using silicon-based complementary metal-oxide semiconductor circuits2,5, Mott-memristor-based negative differential resistance (NDR) devices6,7 and antiambipolar p–n heterojunction8 transistors. However, these artificial neurons are restricted to pure electrical modulation of neural features, do not explore the ion-/neurotransmitter-based modulation mechanisms of real biological neurons and generally operate at time scales and spike voltage swings drastically different from those found in biology. As a result, these circuits do not facilitate integration with biology or direct sensing/processing of biological, chemical or physical stimuli at the neuron and require coupling of additional sensing elements to act as event-based neuromorphic sensing/processing elements9. Organic electrochemical transistors (OECTs) based on mixed ion–electron conducting polymers are attractive in this context due to their sensing capabilities (biological, chemical and physical signals), biocompatibility, biorealistic switching speeds, low operating voltages and coupled ionic–electronic transport properties which are amenable to modulation by external (ion/molecule) dopants10,11. Recently, we observed that rigid conjugated polymers such as the ladder-type poly(benzimidazobenzophenanthroline) (BBL) exhibit a reduction in the electrical conductivity on high electrochemical doping (>0.8 electrons per monomer) due to the formation of multiply charged species with reduced mobility12.
In this article, we utilize this ion-tunable antiambipolarity to emulate the activation and inactivation of sodium channels in biological neurons and hence realize a conductance-based organic electrochemical neuron (c-OECN). This c-OECN can spike at bioplausible frequencies (100 Hz), replicate most neural features and exhibit stochastic response in the presence of noise. Since the antiambipolar element is responsive to ions and biomolecules, we demonstrate that this neuron can be operated as an event-based sensor transducing such biochemical signals to actuate/stimulate the vagus nerve of a mouse, showing the potential for c-OECN-based closed-loop regulation of physiology.
Antiambipolarity in BBL and its modulation
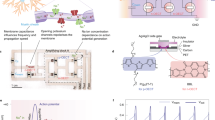
When used as the channel material in OECTs, BBL exhibits a unique, stable and reversible Gaussian-shaped transfer curve (or antiambipolar behaviour) which is similar to voltage-controlled NDR but here instead realized in a three-terminal configuration (Fig. 1a–c and Supplementary Figs. 1–4). When implemented in a circuit, the two sides of this Gaussian current evolution can be analogous to the activated and inactivated states of the voltage-gated sodium channel in the Hodgkin–Huxley neuron model (HH model; Supplementary Note 1)13. Although such antiambipolar behaviour can also be observed in other n- and p-type polymers (Supplementary Figs. 5–7), only BBL with suitable electron affinity (4.15 eV (ref. 14)) and a rigid ladder-like structure composed of double-strand chains linked by condensed π-conjugated units can sustain such high doping levels without any conformational disorder15, enabling reversibility.
a, Structure of BBL. b, Structure of an OECT device. c, The antiambipolar behaviour in BBL resembles the activation and inactivation of sodium channels in a neuron. d–h, Modulation of the antiambipolar behaviour by electrical and chemical means, showing the effects of VDS dependence (d), different gate electrodes (e), ion concentration (f), ion type (g) and different amino acids/neurotransmitters (h). The OECT used in the comparison has a W/L = 40 µm/6 µm and 20-nm-thick BBL except for the higher-current NH4Cl device, which uses a wider channel (W/L = 400 µm/6 µm). A concentration of 100 mM is used for comparing various types of ions. Neurotransmitter and amino acid studies are carried out in 100 mM NaCl. The vertical dashed lines in d–h denote the gate voltage corresponding to the peak drain current (VP). The solid and dashed lines in h denote the forward and reverse scans.
The OECT configuration provides improved control over the antiambipolar response compared with a conventional two-terminal NDR device. For example, applying a higher drain voltage (VDS) increases the peak current, causes a shift in the voltage of the peak current (VP) and results in a Gaussian distribution of a greater full-width at half maximum (Fig. 1d) due to variable doping levels at the drain and source electrodes. The VP and threshold voltage (VT) can also be shifted and controlled by using gate electrodes of appropriate work function (Fig. 1e) and by tuning the concentration of ions in the electrolyte (Fig. 1f). In addition, for a given concentration (100 mM), different ions shift the transfer curve by varying degrees, with Ca2+, Mg2+ and NH4+ showing lower VP than Na+ and K+ (Fig. 1g). Interestingly, various ammonium-based organic cations also lead to unique Gaussian behaviours (Supplementary Fig. 8), unlocking the possibility of chemical-specific responses. Hence it is possible to tune the antiambipolar response using different neurotransmitters and amino acids such as acetylcholine, dopamine, γ-butyric acid (GABA) and glutamine with different configurations of amine groups (Fig. 1h). Adding 3.3 mM acetylcholine to the 100 mM NaCl electrolyte of the OECT does not shift the VP, whereas the same concentration of dopamine, GABA or glutamine causes changes in VP and the channel’s conductance. We attributed this effect to hydrogen-bonding interactions of these molecules with BBL (Supplementary Note 1 and Supplementary Figs. 9–14).
Conductance-based organic electrochemical neuron
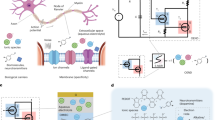
The c-OECN circuit described here uses two OECTs—one Na+-based (Na-OECT) and another K+-based (K-OECT)—coupled to two voltage sources ENa (500 mV) and EK (∼12 to −72 mV) like the two ion channels and batteries in the HH model13,16 (Fig. 2a–d). ENa is applied to the drain of Na-OECT and EK to the source of K-OECT. The switching speed of these OECTs is in the range of 0.5–1 ms (ref. 17) (Supplementary Figs. 15 and 16), which is comparable to the time scales of sodium and potassium channel activation in biological neurons. The K-OECT channel has a thicker BBL film (50 nm compared with 20 nm for the Na-OECT) to allow higher currents through the potassium channel.
a–c, Analogy between biological neurons (a) showing Na+ and K+ channels (b) and the c-OECN circuit with Na+- and K+-based OECTs (c) and their modulation with Ca2+ and neurotransmitters. Cmem is optional in the circuit and can be embedded in the intrinsic capacitance of the OECT. d, The Hodgkin–Huxley circuit of the neuron. e,f, Comparison of the squid axon action potential studied by Hodgkin and Huxley13 (e) and the c-OECN action potential (f). Cmem is the membrane capacitance, Vmem the membrane voltage, Iin the input current and Rdk the resistance which induces delay. EL (RL), EK (RK) and ENa (RNa) represent the leakage, potassium and sodium batteries (resistances) in the HH model. VK and μK are the threshold voltage and mobility of the K-OECT. VNa-s (μNa-s) and VNa-m (μNa-m) are the threshold voltage (mobility) on the low- and high-voltage sides of the antiambipolar transfer curve of Na-OECT representing the single and multiply charged species. INa and IK are the sodium and potassium currents. Part e: copyright 1952 Wiley. Adapted with permission from A.L. Hodgkin, A.F. Huxley, A quantitative description of membrane current and its application to conduction and excitation in nerve, Journal of Physiology, John Wiley and Sons.
A current Iin (in the range 0.5–6 μA) injected into this circuit is integrated by the capacitance Cmem causing the voltage Vmem to increase from its resting value of ∼175 mV. This simultaneously sweeps the voltage on the gate of Na-OECT from ∼1.2 V to ∼0.8 V using an n-type metal-oxide semiconductor-based inverting amplifier (Supplementary Fig. 17) to traverse the antiambipolar transfer curve through VP, producing a spike in current INa1. This current spike charges the capacitor even further, resulting in a rapid increase of Vmem to ∼0.34 V (depolarization). The K-OECT, which turns on after a delay enabled by its intrinsic gate capacitance and resistance Rdk, reaches its peak current after Na-OECT has crossed its maximum at VP. The capacitor is thus discharged through K-OECT, causing the Vmem to drop (repolarization) and traversing the Gaussian response to its initial state back through VP, causing a secondary spike INa2. The secondary spike in Na-OECT (not present in the squid axon action potential; Fig. 2e,f) is unnecessary for spike generation but is unavoidable, hence is entirely discharged by the K-OECT (due to the higher conductivity of K-OECT) not to cause any further voltage increase. Since the current of K-OECT is higher and persists longer, the voltage is brought below the resting value of 175 mV for a brief period (hyperpolarization). All these processes repeat cyclically if the input current remains constant, resulting in continuous action potential generation (tonic spiking). The c-OECN action potential thus shows typical features of a biological action potential, including depolarization, repolarization and hyperpolarization (Fig. 2f, Supplementary Figs. 18–25 and Supplementary Video 1). Supplementary Note 4 provides the circuit analysis of the c-OECN and the SPICE simulation and compares the circuit equations with the HH model. Note that VDS for Na-OECT is not constant because the voltage at the source side is Vmem, which continuously fluctuates. Hence, the effective VDS of Na-OECT is ENa – Vmem. Similarly, Vmem acts at the drain of the K-OECT, and thus its effective VDS is Vmem – Ek.
Features of the c-OECN
The resemblance between the operation of the c-OECN and that of the biological neuron means that several neural features17 can be mimicked by modulating the threshold and currents of OECTs (Fig. 3 and Supplementary Table 1). In the standard configuration, the neuron exhibits tonic spiking, that is, excitability in the presence of a constant input while remaining quiescent otherwise. Tuning the capacitance Cmem and the resistance Rdk can tune the frequency of this spiking. The c-OECN spikes at a frequency of around 5 Hz with a Cmem = 1 μF and Rdk = 470 kΩ (Fig. 3a) and can be increased to reach 45 Hz or 80 Hz (Fig. 3b,c) by excluding external capacitance and then utilizing only the internal capacitance of the OECT. Furthermore, the n-type metal-oxide semiconductor-based inverting amplifier can be replaced with an OECT-based amplifier to achieve spiking at 100 Hz (Supplementary Figs. 26 and 27). Hence, biorealistic frequencies can be achieved with a neuron based on three OECTs. For ease of measurement of neural features with various pulsed inputs, we used the lower-frequency c-OECN (~5 Hz) by employing Cmem = 1 μF. The peak power (energy) consumption of the circuit spiking at 80 Hz is around 60 μW (175 nJ per spike). Supplementary Note 5 discusses strategies to lower energy consumption.
a–p, Tonic spiking (a), higher-frequency tonic spiking by modulating Rdk and Cmem (45 Hz, b; 80 Hz, c), latency (d), integration (e), refractoriness (f), resonance (g), threshold variability (h), rebound spike (i), accommodation (j), class 3 spiking—phasic bursting and spiking (k), class 1 spiking (l), class 2 spiking (m), stochastic spiking with noisy input (n), calcium-based modulation from class 1 to class 3 spiking (o) and modulation of spiking using the amino acid glutamine (p). The x axis of all the graphs represents time. Vmem and Iin are the membrane voltage and input current. The parameters used to enable these behaviours are listed in Supplementary Table 1.
Most neural features such as tonic spiking, latency, subthreshold oscillations, integration, refractoriness, resonance, threshold variability, rebound spiking, accommodation, phasic spiking, phasic bursting, and class 1 and class 2 excitability can be demonstrated using this circuit (Fig. 3d–m). Each of these has a specific function or serves as a mathematical operator in a neuron (Supplementary Note 6). Switching from class 1 (input-strength-dependent excitability) to class 2 (spiking only at high current inputs with a high frequency) and class 3 spiking (spiking/bursting only at the beginning of input) can be enabled by simple tuning of the threshold of the K-OECT (by changing Ek) to modify the relative timing of it turning on. For example, for the c-OECN demonstrated here, an Ek of −50 mV results in a class 1 spiking neuron, while causing K-OECT to turn on earlier by changing Ek to −65 mV changes the behaviour to class 2 spiking, and increasing it to a more negative value of −70 mV (−72 mV) results in phasic bursting (phasic spiking) which is class 3 behaviour. Incorporating additional channels in the circuit similar to the Ca2+ modulated channels in biological neurons18 may enable other missing features achievable by biological neurons such as tonic bursting, mixed-mode spiking, spike frequency adaptation, bistability and inhibition-induced spiking17.
The c-OECN can also exhibit input-noise-dependent stochasticity or spike skipping, like the biological neurons19. The class 2 neuron shown in Fig. 3n does not spike at an input current of 2 μA. However, when very low noise is superimposed on this input, keeping the average current the same, it starts spiking at a particular frequency—increasing the input noise results in random skipping of spikes while keeping this base frequency constant. A similar mechanism is observed in biological neurons, for example, in mammalian cold thermoreceptors19 where temperature-induced noise causes stochasticity in spiking and is used to extend the range of encodable stimuli. Such stochastic spiking can also enable probabilistic neural sampling and finds application in spike-based Bayesian learning and inference20.
Modulation using (bio)chemical signals and biointegration
The unique feature of the c-OECN compared to other conductance-based circuit realizations of neurons is that it can be controlled using secondary ions such as Ca2+ and neurotransmitters as they can affect the VP and the maximum current of the Gaussian transfer curve. In biological neurons, Ca2+ plays crucial roles in regulating neural activity by modifying the opening and closing of sodium and potassium channels, stimulating the release of neurotransmitters leading to synaptic plasticity, and even regulating metabolism and cell growth21. Inspired by this, we tried to modulate the potassium channel of the c-OECN using external Ca2+ ions. Incorporating Ca2+ ions into the electrolyte of the K-OECT shifts its threshold VK towards lower values. This is equivalent to VK modulation by altering EK as described above and hence creates the same effect, that is, a shift from class 1 spiking to phasic bursting and finally phasic spiking on a slow increase of Ca2+ concentration (Fig. 3o). Such a transition is similar to the case in biological neurons, where the generation of phasic firing is known to be Ca2+ ion concentration dependent22. In addition to ions, biological neurons are also affected by the presence of neurotransmitters and amino acids. For example, GABA is an inhibitory neurotransmitter in the brain23 and inhibits the generation of action potentials by increasing chloride or potassium ion conductance and hyperpolarizing the membrane. Here we coupled GABA to the Na-OECT, and since GABA reduces the maximum current of the Na-OECT and shifts its VP towards lower values, the sodium current spike induced depolarization, and hence spiking is instantly inhibited in the c-OECN, thus enabling neurotransmitter-induced modulation of spiking similar to biology (Supplementary Fig. 41). Similarly, the c-OECN can be controlled by the amino acid glutamine such that it stops spiking above biologically relevant concentrations of around 1 mM (ref. 24) (Fig. 3p).
To further illustrate the possibility of using c-OECNs in sensing, biointegration and nerve activation, we demonstrate event-based sensing of Na+ and glutamine (Fig. 4a–f) at the neuron combined with stimulation of the right cervical vagus nerve of a mouse to control its heart rate. Vagus nerve stimulation (VNS) is used as a therapeutic intervention for treating depression, controlling epileptic seizures and in clinical trials for treating chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease25,26. Experimental animal models (commonly genetic mouse models) are often used to study the physiological effects and mechanisms of VNS and to estimate its therapeutic potential. Here we coupled the c-OECN with a mouse’s right cervical vagus nerve, which innervates the sinoatrial node where the pacemaker cells for heart rate are located, using a cuff electrode27 (Supplementary Fig. 43), and monitored the heart rate. We observed a 4.5% reduction in heart rate in response to an increased Na+ concentration sensed at the Na-OECT, consistent with electrical activation of the right cervical vagus nerve (Fig. 4g–k). As discussed previously, the NaCl concentration can substantially shift the VP of the Gaussian transfer curve (Fig. 1f) of the Na-OECT to modulate the spiking of the c-OECN, thus enabling event-based ion sensing at the neuron without external sensors (Fig. 4a–d). Sensing Na+ has high clinical significance in detecting diseases such as cystic fibrosis, where the sweat Na+ and Cl− concentrations can increase to high levels (>60 mM) compared with baseline (<30 mM)28. The c-OECN can be made to spike only at a high NaCl concentration (>25 mM) relevant to this condition (by choosing appropriate values of VK, VNa and inverter threshold) and resulting in an actuation; here, for example, the VNS-based control of heart rate. The sensing capability of the c-OECN is not limited to ions. It can also be made to spike only at either a low (<900 μM) or high (>1,800 μM) glutamine concentration in the biologically relevant range and combined with stimulation of the vagus nerve. The demonstration of sensor-triggered vagus nerve activation does not imply that a new therapeutic means is developed in this study but shows the potential for future c-OECN-based closed-loop regulation of physiology. Considering the key role the vagus nerve plays in regulating homeostasis, including immune-system and metabolic control29, VNS triggered by event-based internal sensing has interesting potential therapeutic applications, for example, in improving the regulation of cytokine release or glucose levels to treat autoimmune or metabolic diseases. This notion may be extended to sensor-based regulation of other aspects of normal physiology and pathological conditions through peripheral nerve activation, such as VNS and voltage-triggered drug delivery.
a–d, Modulation of spiking of c-OECN based on NaCl concentrations of 12.5 mM (a), 25 mM (b), 50 mM (c) and 100 mM (d). e,f, A neuron optimized to spike at low glutamine concentrations (<900 μM) (e) and at high glutamine concentrations (>1,800 μM) (f). g, The c-OECN circuit showing sensing of Na+ ions at the Na-OECT and integration with the vagus nerve using an OECT-based amplifier and cuff electrodes. h,i, The amplifier output at a low NaCl concentration of 25 mM (h) and the corresponding heart rate variation (i). j,k, The amplifier output at a high NaCl concentration of 100 mM (j) and the corresponding heart rate variation (k). The black horizontal dashed line in k represents the baseline heart rate. The purpose of this demonstration is to show the potential of c-OECN to sense biochemical signals and interface with nerves and does not imply that new therapeutic means are developed.
In conclusion, we demonstrated a biorealistic OECN based on highly tunable, stable and reversible antiambipolar behaviour in BBL-based OECTs. Two OECTs modulated by Na+ and K+ ions resemble the voltage-gated ion channels in biological neurons, enabling various neural features and facile sensing and integration with the vagus nerve of a mouse. A comparison of the c-OECN with other neuron technologies is provided in Supplementary Table 2. The intrinsic capacitance of the OECTs and the biorealistic switching speeds circumvent the need for additional capacitors required in silicon-based circuits to spike at biologically plausible frequencies for interaction with real-world events and biological neurons30. Alternative implementations using Mott-memristors that can exhibit similar features are inherently faster than biology (ns to μs) and are associated with unfavourable temperature increases on operation, making them unsuitable for biointegration. In addition, using single-polymer material to achieve Gaussian behaviour similar to NDR averts complex fabrication strategies and helps achieve lower device dimensions compared with the heterojunction approach in 2D materials or p–n junction polymers. The ion-, amino acid- and neurotransmitter-based modulation in this c-OECN is unprecedented and may be extendable to other biomolecules that can interact with BBL. Although the sensing of molecules is done externally in the current study, it demonstrates the capability of tuning the c-OECN to respond to a specific concentration of biochemical signal by simple modulation of voltages in the circuit to enable event-based sensing. Similar stable ladder-like conjugated polymers functionalized to interact with specific biomolecules are a possible way forward to realize intelligent closed-loop event-based internal sensing and feedback neuromorphic biomedical systems and future brain–machine interfaces.
Methods
Materials
Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS, Clevios PH1000) was purchased from Heraeus Holding. Naphthalenetetracarboxylic dianhydride (NDA), 1,2,4,5-tetraaminobenzene tetrahydrochloride (TABH), poly(phosphoric acid) (PPA), methanesulfonic acid (MSA), chloroform, 1,2-dichlorobenzene, ethylene glycol, (3-glycidyloxypropyl)trimethoxysilane and 4-dodecylbenzenesulfonic acid were purchased from Sigma-Aldrich. BBL (η = 6.3 dl g−1 in MSA at 30 °C, Mw = 35 kDa) was made by polycondensation of NDA and TABH in PPA at elevated temperatures17,31.
Thin-film casting
BBL was dissolved in MSA at 100 °C for 12 h, followed by cooling to room temperature to obtain the BBL–MSA solution. This solution was spin-coated (1,000 r.p.m., 60 s, acceleration 1,000 r.p.m. s−1) on OECT substrates. The residual MSA in the films was removed by immersing them in deionized water, followed by drying in nitrogen flow. PEDOT:PSS, ethylene glycol, (3-glycidyloxypropyl)trimethoxysilane and 4-dodecylbenzenesulfonic acid were mixed in the volume ratio of 100:5:1:0.1 and sonicated for 10 min. This solution was spin-coated (2,000 r.p.m., acceleration 2,000 r.p.m. s−1, 60 s) on OECT substrates and annealed at 120 °C for 1 min for crosslinking PEDOT:PSS.
OECT fabrication and testing
OECTs were fabricated according to a previous protocol17,32. Four-inch glass wafers were cleaned with acetone, deionized water and isopropyl alcohol and then dried using nitrogen. Electrodes (5 nm chromium and 50 nm gold) were thermally evaporated and then patterned by photolithography. A layer of parylene carbon (PaC, 1 µm) was then deposited in the presence of 3-(trimethoxysilyl)propyl methacrylate (A-174 Silane) (to increase adhesion). This forms an insulating layer to prevent unwanted capacitive effects at the electrode–electrolyte interface. After that, an antiadhesive layer of industrial surfactant (2% Micro-90) was spin-coated, and a sacrificial 2-µm-thick layer of PaC was deposited. A 5-µm-thick AZ10XT520CP positive photoresist was then spin-coated on this layer. This protects the PaC layers from the following plasma reactive ion etching (RIE) step (150 W, 500 sccm O2, 100 sccm CF4, 380 s). Another photolithographic patterning was performed to define the contact pads and the OECT channel, and the AZ developer was applied to the photoresist. A plasma RIE was carried out to remove the organic materials (photoresist and PaC), exposing the OECT channel area and the contact pads. The remaining surface remained covered with layers of PaC. This was followed by patterning the OECT channel to obtain a width/length (W/L) = 40 µm/6 µm or 80 µm/6 µm (for Na-OECTs), and 400 µm/6 µm (for K-OECTs). A 20-nm-thick (Na-OECT) or 50-nm-thick (K-OECT) film was obtained by spin-coating the BBL–MSA solution described previously. The sacrificial parylene layer was then peeled off to remove the unwanted BBL film outside the electrode area. This leaves separated pieces of semiconductor film confined to the wells, connecting the OECT source/drain electrodes. Ag/AgCl paste was drop-cast on the substrate to form a 1-μm-thick, 9 mm2 square gate electrode. OECTs based on PEDOT:PSS, P(g42T-T) and p(g7NC10N) were also made using the same procedure. The electrolyte for all the OECT measurements was 0.1 M NaCl aqueous solution unless otherwise specified. The OECTs were characterized using a Keithley 4200A-SCS.
SPICE simulation
The SPICE models of K-OECT and Na-OECT were created in B2 SPICE (EMAG Technologies). These models simulate the spiking features of c-OECNs. The details of the simulation are presented in Supplementary Note 4.
Cuff electrode fabrication and interfacing with the vagus nerve
To interface with the mouse’s vagus nerve, a flexible array of electrodes was fabricated based on a previous protocol33. Eight stimulation electrodes (450 µm × 200 µm in dimensions) in a 2 × 4 arrangement were included in the array. The substrate and insulation layers consisted of flexible PaC which allow the device to conformally wrap around the nerve, and thus to serve as a cuff-electrode-style interface. The first stage of the microfabrication process consisted of a cleaning procedure for glass microscope slides using ultrasonication in a dilute Hellmanex soap solution (2 vol% in deionized water), then in acetone, followed by isopropanol. A flexible PaC layer (2 µm) was then deposited using chemical vapour deposition (Diener Electronic) on the glass carrier substrates. Photolithographic patterning of metal interconnects (80 nm gold and 5 nm titanium adhesion layer) was carried out through a lift-off process using the negative photoresist AZ nLof 2070 and a MA6 Suss mask aligner with an i-line filter. These metal patterns provide the electrode contact surfaces and an electrical connection between the electrodes and the back-end contact pads. Following lift-off, a 2 min oxygen plasma process at a power of 50 W was performed before the deposition of an insulating PaC layer (1.5 µm) on the metal electrode lines (with A-174 adhesion enhancer in the deposition chamber). An AZ 10XT photoresist etch mask was patterned, and RIE was performed (O2/CF4 gases, 150 W) to define the shape of the probes. Acetone and isopropanol rinses were used to remove the photoresist etch mask. Next, a dilute soap antiadhesion layer (2.5 vol% in deionized water) was spin-coated at 1,000 r.p.m. on the sample surface. A thick sacrificial PaC layer (2 μm) was then deposited and the above RIE etch process was used to define the surface of the electrode and back-end contact openings. A conductive polymer electrode coating was spin-coated onto the substrates, consisting of a PEDOT:PSS-based dispersion, 5 wt% ethylene glycol, 0.1 wt% dodecyl benzene sulfonic acid and 1 wt% of (3-glycidyloxypropyl)trimethoxysilane. A soft bake was carried out at 100 °C for 60 s, followed by peel-off of the sacrificial PaC layer. Finally, a 45 min annealing process at 140 °C was used to crosslink the conducting polymer film, and the individual arrays were removed from the glass substrates using deionized water to assist the process.
To provide electrical connection to the arrays, custom adaptors were created using zero-insertion-force clips (ZIF-Clip) mounted on printed circuit boards. Back-end wiring provided the possibility for connection to an Intan 16-channel head stage of an Intan RHS 128 channel stimulation/recording controller (Intan Technologies) with software that helps verify good contact and electrochemical impedance values. When interfacing the c-OECN and the right vagus nerve, four neighbouring electrodes were shorted together and connected to the output of the c-OECN.
C57BL/6J male mice, 10–12 weeks of age, purchased from Charles River Laboratories, were used in experiments investigating the interface with the vagus nerve. Mice were housed under a 12 h light/dark cycle with adequate access to food and water. The approval for this experimental protocol was provided by the regional Stockholm Animal Research Ethics Committee (Stockholm, Sweden). The surgery used to isolate the vagus nerve has been described previously34. In brief, mice were anaesthetized with isoflurane and an equal mixture of air and oxygen. They were then placed in the supine position, and a ventral midline cervical incision was made to expose subcutaneous tissues and the mandibular salivary glands. After separation using blunt dissection, proper exposure revealed the right neurovascular bundle containing the cervical vagus nerve and carotid artery. The vagus nerve was dissected from the vasculature and isolated before immobilizing with a suture to facilitate electrode placement.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the main data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data are provided with this paper.
References
Purves, D. et al. (eds). Neuroscience 3rd edn. (Sinauer Associates Inc., 2004).
Indiveri, G. et al. Neuromorphic silicon neuron circuits. Front. Neurosci. 5, 73 (2011).
Harikesh, P. C. et al. Organic electrochemical neurons and synapses with ion mediated spiking. Nat. Commun. 13, 901 (2022).
Hosseini, M. J. M. et al. Organic electronics Axon–Hillock neuromorphic circuit: towards biologically compatible, and physically flexible, integrate-and-fire spiking neural networks. J. Phys. Appl. Phys. 54, 104004 (2020).
Mahowald, M. & Douglas, R. A silicon neuron. Nature 354, 515–518 (1991).
Pickett, M. D., Medeiros-Ribeiro, G. & Williams, R. S. A scalable neuristor built with Mott memristors. Nat. Mater. 12, 114–117 (2013).
Yi, W. et al. Biological plausibility and stochasticity in scalable VO2 active memristor neurons. Nat. Commun. 9, 4661 (2018).
Beck, M. E. et al. Spiking neurons from tunable Gaussian heterojunction transistors. Nat. Commun. 11, 1565 (2020).
Covi, E. et al. Adaptive extreme edge computing for wearable devices. Front. Neurosci. 15, 611300 (2021).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 1–14 (2018).
Berggren, M. et al. Ion electron–coupled functionality in materials and devices based on conjugated polymers. Adv. Mater. 31, 1805813 (2019).
Xu, K. et al. On the origin of Seebeck coefficient inversion in highly doped conducting polymers. Adv. Funct. Mater. 32, 2112276 (2022).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Xu, K. et al. Ground-state electron transfer in all-polymer donor–acceptor heterojunctions. Nat. Mater. 19, 738–744 (2020).
Fazzi, D. & Negri, F. Addressing the elusive polaronic nature of multiple redox states in a π-conjugated ladder-type polymer. Adv. Electron. Mater. 7, 2000786 (2021).
Hodgkin, A. L. & Huxley, A. F. Action potentials recorded from inside a nerve fibre. Nature 144, 710–711 (1939).
Wu, H.-Y. et al. Influence of molecular weight on the organic electrochemical transistor performance of ladder-type conjugated polymers. Adv. Mater. 34, 2106235 (2022).
Izhikevich, E. M. Which model to use for cortical spiking neurons? IEEE Trans. Neural Netw. 15, 1063–1070 (2004).
Gerstner, W., Kistler, W. M., Naud, R. & Paninski, L. Neuronal Dynamics: From Single Neurons to Networks and Models of Cognition (Cambridge Univ. Press, 2014).
Longtin, A. & Hinzer, K. Encoding with bursting, subthreshold oscillations, and noise in mammalian cold receptors. Neural Comput. 8, 215–255 (1996).
Buesing, L., Bill, J., Nessler, B. & Maass, W. Neural dynamics as sampling: a model for stochastic computation in recurrent networks of spiking neurons. PLoS Comput. Biol. 7, e1002211 (2011).
Simons, T. J. B. Calcium and neuronal function. Neurosurg. Rev. 11, 119–129 (1988).
Suzuki, S. & Rogawski, M. A. T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proc. Natl Acad. Sci. USA 86, 7228–7232 (1989).
McCormick, D. A. GABA as an inhibitory neurotransmitter in human cerebral cortex. J. Neurophysiol. 62, 1018–1027 (1989).
Ben-Menachem, E. et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 20, 221–227 (1995).
Johnson, R. L. & Wilson, C. G. A review of vagus nerve stimulation as a therapeutic intervention. J. Inflamm. Res. 11, 203–213 (2018).
Caravaca, A. S. et al. Vagus nerve stimulation promotes resolution of inflammation by a mechanism that involves Alox15 and requires the α7nAChR subunit. Proc. Natl Acad. Sci. USA 119, e2023285119 (2022).
Donahue, M. et al. Wireless optoelectronic devices for vagus nerve stimulation in mice. J. Neural Eng. 19, 066031 (2022).
Emaminejad, S. et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl Acad. Sci. USA 114, 4625–4630 (2017).
Pavlov, V. A. & Tracey, K. J. Bioelectronic medicine: preclinical insights and clinical advances. Neuron 110, 3627–3644 (2022).
Arnold, F. E. & Van Deusen, R. L. Preparation and properties of high molecular weight, soluble oxobenz[de] imidazobenzimidazoisoquinoline ladder polymer. Macromolecules 2, 497–502 (1969).
Braendlein, M., Lonjaret, T., Leleux, P., Badier, J.-M. & Malliaras, G. G. Voltage amplifier based on organic electrochemical transistor. Adv. Sci. 4, 1600247 (2017).
Donahue, M. J. et al. Multimodal characterization of neural networks using highly transparent electrode arrays. eNeuro 5, ENEURO.0187-18.2018 (2018).
Caravaca, A. S. et al. An effective method for acute vagus nerve stimulation in experimental inflammation. Front. Neurosci. 13, 877 (2019).
Acknowledgements
We thank R. Kroon for synthesizing P(g42T-T) and I. McCulloh for providing p(g7NC10N) which are used to compare antiambipolar behaviour. We thank K. Xu for discussing antiambipolar behaviour in BBL. This work was financially supported by the Knut and Alice Wallenberg Foundation (2021.0058, 2016.0351, WWSC2.0), the Swedish Research Council (2020-03243, 2020-02583), Olle Engkvists Stiftelse (204-0256), VINNOVA (2020-05223), the European Commission through the FET-OPEN project MITICS (GA-964677), the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO-Mat-LiU 2009-00971) and the Swedish Heart–Lung Foundation (20210524).
Funding
Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
P.C.H., D.T. and S.F. conceived and designed the experiments. P.C.H. and D.T. fabricated and tested the circuits and analysed the data. C.-Y.Y. and H.-Y.W. performed the synthesis of materials, fabrication and characterization of the OECTs for the circuit. C.-Y.Y. and J.-D.H. compared the antiambipolar behaviour in various polymers. S.Z. studied the effect of ions on antiambipolar behaviour. M.J.D. designed and fabricated the cuff electrodes. M.J.D., A.S.C. and P.S.O. interfaced the neuron with the vagus nerve. D.T. designed the circuit and conducted the simulations. P.C.H., D.T., M.B. and S.F. wrote the manuscript. All authors contributed to the discussion and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–45 and Tables 1 and 2.
Supplementary Video 1
The operation mechanism of the c-OECN showing the states of various components with time.
Source data
Source Data Fig. 1 to 4
Source data for Figs. 1 to 4 in a single Excel file.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Harikesh, P.C., Yang, CY., Wu, HY. et al. Ion-tunable antiambipolarity in mixed ion–electron conducting polymers enables biorealistic organic electrochemical neurons. Nat. Mater. 22, 242–248 (2023). https://doi.org/10.1038/s41563-022-01450-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01450-8
This article is cited by
-
Understanding asymmetric switching times in accumulation mode organic electrochemical transistors
Nature Materials (2024)
-
Potential and challenges of computing with molecular materials
Nature Materials (2024)
-
A modular organic neuromorphic spiking circuit for retina-inspired sensory coding and neurotransmitter-mediated neural pathways
Nature Communications (2024)
-
Monolithically integrated high-density vertical organic electrochemical transistor arrays and complementary circuits
Nature Electronics (2024)
-
Designing organic mixed conductors for electrochemical transistor applications
Nature Reviews Materials (2024)