Abstract
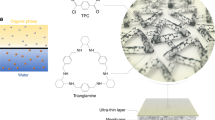
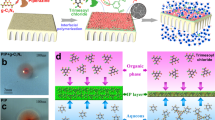
The development of membranes that block solutes while allowing rapid water transport is of great importance. The microstructure of the membrane needs to be rationally designed at the molecular level to achieve precise molecular sieving and high water flux simultaneously. We report the design and fabrication of ultrathin, ordered conjugated-polymer-framework (CPF) films with thicknesses down to 1 nm via chemical vapour deposition and their performance as separation membranes. Our CPF membranes inherently have regular rhombic sub-nanometre (10.3 × 3.7 Å) channels, unlike membranes made of carbon nanotubes or graphene, whose separation performance depends on the alignment or stacking of materials. The optimized membrane exhibited a high water/NaCl selectivity of ∼6,900 and water permeance of ∼112 mol m−2 h−1 bar−1, and salt rejection >99.5% in high-salinity mixed-ion separations driven by osmotic pressure. Molecular dynamics simulations revealed that water molecules quickly and collectively pass through the membrane by forming a continuous three-dimensional network within the hydrophobic channels. The advent of ordered CPF provides a route towards developing carbon-based membranes for precise molecular separation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and Supplementary Information. Source data are provided with this paper.
References
Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605 (2000).
Gouaux, E. & MacKinnon, R. Principles of selective ion transport in channels and pumps. Science 310, 1461–1465 (2005).
Shen, J., Liu, G., Han, Y. & Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Li, H. et al. Na+-gated water-conducting nanochannels for boosting CO2 conversion to liquid fuels. Science 367, 667–671 (2020).
Dakhchoune, M. et al. Gas-sieving zeolitic membranes fabricated by condensation of precursor nanosheets. Nat. Mater. 20, 362–369 (2021).
Liu, G. et al. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 17, 283–289 (2018).
Tan, R. et al. Hydrophilic microporous membranes for selective ion separation and flow-battery energy storage. Nat. Mater. 19, 195–202 (2020).
Di Vincenzo, M. et al. Biomimetic artificial water channel membranes for enhanced desalination. Nat. Nanotech. 16, 190–196 (2021).
Song, W. et al. Artificial water channels enable fast and selective water permeation through water-wire networks. Nat. Nanotech. 15, 73–79 (2020).
Zhang, W.-H. et al. Graphene oxide membranes with stable porous structure for ultrafast water transport. Nat. Nanotech. 16, 337–343 (2021).
Yang, Y. et al. Large-area graphene-nanomesh/carbon-nanotube hybrid membranes for ionic and molecular nanofiltration. Science 364, 1057–1062 (2019).
Ding, L. et al. Effective ion sieving with Ti3C2Tx MXene membranes for production of drinking water from seawater. Nat. Sustain. 3, 296–302 (2020).
Ali, Z. et al. Finely tuned submicroporous thin-film molecular sieve membranes for highly efficient fluid separations. Adv. Mater. 32, 2001132 (2020).
Lu, J. et al. Efficient metal ion sieving in rectifying subnanochannels enabled by metal–organic frameworks. Nat. Mater. 19, 767–774 (2020).
Marcotte, A., Mouterde, T., Niguès, A., Siria, A. & Bocquet, L. Mechanically activated ionic transport across single-digit carbon nanotubes. Nat. Mater. 19, 1057–1061 (2020).
Feng, J. et al. Single-layer MoS2 nanopores as nanopower generators. Nature 536, 197 (2016).
Werber, J. R., Osuji, C. O. & Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 1, 16018 (2016).
Joseph, S. & Aluru, N. R. Why are carbon nanotubes fast transporters of water? Nano Lett. 8, 452–458 (2008).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357, 792–796 (2017).
Secchi, E. et al. Massive radius-dependent flow slippage in carbon nanotubes. Nature 537, 210 (2016).
Gopinadhan, K. et al. Complete steric exclusion of ions and proton transport through confined monolayer water. Science 363, 145–148 (2019).
Nessim, G. D. et al. Tuning of vertically-aligned carbon nanotube diameter and areal density through catalyst pre-treatment. Nano Lett. 8, 3587–3593 (2008).
Shen, J. et al. Subnanometer two-dimensional graphene oxide channels for ultrafast gas sieving. ACS Nano 10, 3398–3409 (2016).
Qiu, H., Xue, M., Shen, C., Zhang, Z. & Guo, W. Graphynes for water desalination and gas separation. Adv. Mater. 31, 1803772 (2019).
Moreno, C. et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018).
Xu, C. et al. Two-dimensional polyphenylene networks with tunable micropores for hydrogen storage. ACS Sustain. Chem. Eng. 7, 18341–18349 (2019).
Bartolomei, M. et al. Graphdiyne pores: ‘ad hoc’ openings for helium separation applications. J. Phys. Chem. C. 118, 29966–29972 (2014).
Bartolomei, M. et al. Penetration barrier of water through graphynes’ pores: first-principles predictions and force field optimization. J. Phys. Chem. Lett. 5, 751–755 (2014).
Yu, H., Xue, Y. & Li, Y. Graphdiyne and its assembly architectures: synthesis, functionalization, and applications. Adv. Mater. 31, 1803101 (2019).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Galeotti, G. et al. Synthesis of mesoscale ordered two-dimensional π-conjugated polymers with semiconducting properties. Nat. Mater. 19, 874–880 (2020).
Grossmann, L. et al. On-surface photopolymerization of two-dimensional polymers ordered on the mesoscale. Nat. Chem. 13, 730–736 (2021).
Zhou, J., Li, J., Liu, Z. & Zhang, J. Exploring approaches for the synthesis of few-layered graphdiyne. Adv. Mater. 31, 1803758 (2019).
He, J. et al. Hydrogen substituted graphdiyne as carbon-rich flexible electrode for lithium and sodium ion batteries. Nat. Commun. 8, 1172 (2017).
Zhang, Y. Q. et al. Homo-coupling of terminal alkynes on a noble metal surface. Nat. Commun. 3, 1286 (2012).
Radha, B. et al. Molecular transport through capillaries made with atomic-scale precision. Nature 538, 222–225 (2016).
Liu, R. et al. Chemical vapor deposition growth of linked carbon monolayers with acetylenic scaffoldings on silver foil. Adv. Mater. 29, 1604665 (2017).
Chen, T.-A. et al. Wafer-scale single-crystal hexagonal boron nitride monolayers on Cu (111). Nature 579, 219–223 (2020).
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotech. 12, 546–550 (2017).
Qian, Y. et al. Enhanced ion sieving of graphene oxide membranes via surface amine functionalization. J. Am. Chem. Soc. 143, 5080–5090 (2021).
Ries, L. et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 18, 1112–1117 (2019).
Sapkota, B. et al. High permeability sub-nanometre sieve composite MoS2 membranes. Nat. Commun. 11, 2747 (2020).
O’Hern, S. C. et al. Nanofiltration across defect-sealed nanoporous monolayer graphene. Nano Lett. 15, 3254–3260 (2015).
Lokesh, M., Youn, S. K. & Park, H. G. Osmotic transport across surface functionalized carbon nanotube membrane. Nano Lett. 18, 6679–6685 (2018).
Jian, M. et al. Ultrathin water-stable metal-organic framework membranes for ion separation. Sci. Adv. 6, eaay3998 (2020).
Liu, H., Wang, H. & Zhang, X. Facile fabrication of freestanding ultrathin reduced graphene oxide membranes for water purification. Adv. Mater. 27, 249–254 (2015).
Ma, D., Peh, S. B., Han, G. & Chen, S. B. Thin-film nanocomposite (TFN) membranes incorporated with super-hydrophilic metal–organic framework (MOF) UiO-66: toward enhancement of water flux and salt rejection. ACS Appl. Mater. Interfaces 9, 7523–7534 (2017).
Ma, N., Wei, J., Liao, R. & Tang, C. Y. Zeolite–polyamide thin film nanocomposite membranes: towards enhanced performance for forward osmosis. J. Membr. Sci. 405–406, 149–157 (2012).
Ren, J. & McCutcheon, J. R. A new commercial thin film composite membrane for forward osmosis. Desalination 343, 187–193 (2014).
Bregante, D. T. et al. The shape of water in zeolites and its impact on epoxidation catalysis. Nat. Catal. 4, 797–808 (2021).
Yuan, Y. D. et al. Porous organic cages as synthetic water channels. Nat. Commun. 11, 4927 (2020).
Villalobos, L. F. et al. Bottom-up synthesis of graphene films hosting atom-thick molecular-sieving apertures. Proc. Natl Acad. Sci. USA 118, e2022201118 (2021).
Wu, M. et al. Seeded growth of large single-crystal copper foils with high-index facets. Nature 581, 406–410 (2020).
Acknowledgements
The financial support for this work was provided by Baseline Funds (BAS/1/1372-01-01) to Y.H. from King Abdullah University of Science and Technology (KAUST), to J.J. from the A*STAR AME IRG Grant (A20E5c0092), the Ministry of Education of Singapore and the National University of Singapore (R-279-000-598-114 and R-279-000-574-114). This work was also partially supported by the National Key Research and Development Project of China (2022YFE0113800). V.T. and Y.C. are indebted to the support from the KAUST Office of Sponsored Research (OSR) under award number OSR-2018-CARF/CCF-3079. V.T. acknowledges support from KAUST Solar Center (KSC). This research used resources of the Core Laboratories of KAUST. We acknowledge helpful discussions with Q. Li and J. Wang from Soochow University; J. Yin, Y. Wan and L. Chen from KAUST, and Y. Zhang from Nanjing Tech University.
Author information
Authors and Affiliations
Contributions
Y.H., V.T. and J.J. conceived and designed the experiments. J.S, Y.C., C.Z., C.C., L.L., Y.M., X.D., J.-H.F., C.-C.T. and J.L. performed the synthesis of CPF materials, characterizations and transport measurements. W.W. performed the molecular dynamics simulations and K.Y. conducted the density-functional theory calculations. Y.W., I.P., X.Z. and L.L. provided constructive opinion and suggestions. All authors were involved in the analysis and discussion of the results. Y.H., J.J., V.T. and I.P. wrote the manuscript and all authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Damien Voiry, Xiwang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–34, Tables 1–4, notes 1–3 and references 1–83.
Supplementary Video 1
Water molecules moving in the channels to form a regular 3D network inside the membrane.
Source data
Source Data Fig. 1
Source Data for Figures 1d–1g.
Source Data Fig. 2
Source Data for Figure 2d.
Source Data Fig. 3
Source Data for Figure 3a.
Source Data Fig. 4
Source Data for Figures 4c, 4e and 4f.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Cai, Y., Zhang, C. et al. Fast water transport and molecular sieving through ultrathin ordered conjugated-polymer-framework membranes. Nat. Mater. 21, 1183–1190 (2022). https://doi.org/10.1038/s41563-022-01325-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01325-y
This article is cited by
-
Extreme Li-Mg selectivity via precise ion size differentiation of polyamide membrane
Nature Communications (2024)
-
Sub-8 nm networked cage nanofilm with tunable nanofluidic channels for adaptive sieving
Nature Communications (2024)
-
Bio-inspired design of next-generation ultrapermeable membrane systems
npj Clean Water (2024)
-
Ptychographic X-ray computed tomography of porous membranes with nanoscale resolution
Communications Materials (2023)
-
Eliminating lattice defects in metal–organic framework molecular-sieving membranes
Nature Materials (2023)