Abstract
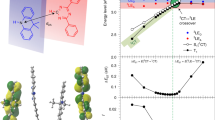
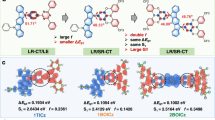
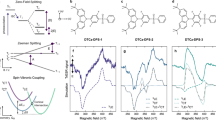
Thermally activated delayed fluorescence enables organic semiconductors with charge transfer-type excitons to convert dark triplet states into bright singlets via reverse intersystem crossing. However, thus far, the contribution from the dielectric environment has received insufficient attention. Here we study the role of the dielectric environment in a range of thermally activated delayed fluorescence materials with varying changes in dipole moment upon optical excitation. In dipolar emitters, we observe how environmental reorganization after excitation triggers the full charge transfer exciton formation, minimizing the singlet–triplet energy gap, with the emergence of two (reactant-inactive) modes acting as a vibrational fingerprint of the charge transfer product. In contrast, the dielectric environment plays a smaller role in less dipolar materials. The analysis of energy–time trajectories and their free-energy functions reveals that the dielectric environment substantially reduces the activation energy for reverse intersystem crossing in dipolar thermally activated delayed fluorescence emitters, increasing the reverse intersystem crossing rate by three orders of magnitude versus the isolated molecule.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the plots within this paper are available at the University of Cambridge Repository: https://doi.org/10.17863/CAM.85068.
References
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photon. 8, 326–332 (2014).
Lin, T.-A. et al. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated delayed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 28, 6976–6983 (2016).
Czerwieniec, R., Yu, J. & Yersin, H. Blue-light emission of Cu(I) complexes and singlet harvesting. Inorg. Chem. 50, 8293–8301 (2011).
Dias, F. B. et al. Triplet harvesting with 100% efficiency by way of thermally activated delayed fluorescence in charge transfer OLED emitters. Adv. Mater. 25, 3707–3714 (2013).
Liu, Y., Li, C., Ren, Z., Yan, S. & Bryce, M. R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 3, 18020 (2018).
Wu, T.-L. et al. Diboron compound-based organic light-emitting diodes with high efficiency and reduced efficiency roll-off. Nat. Photon. 12, 235–240 (2018).
Etherington, M. K., Gibson, J., Higginbotham, H. F., Penfold, T. J. & Monkman, A. P. Revealing the spin–vibronic coupling mechanism of thermally activated delayed fluorescence. Nat. Commun. 7, 13680 (2016).
Gibson, J., Monkman, A. P. & Penfold, T. J. The importance of vibronic coupling for efficient reverse intersystem crossing in thermally activated delayed fluorescence molecules. ChemPhysChem 17, 2956–2961 (2016).
Noda, H. et al. Critical role of intermediate electronic states for spin–flip processes in charge-transfer-type organic molecules with multiple donors and acceptors. Nat. Mater. 18, 1084–1090 (2019).
Drummond, B. H. et al. Electron spin resonance resolves intermediate triplet states in delayed fluorescence. Nat. Commun. 12, 4532 (2021).
dos Santos, P. L., Ward, J. S., Bryce, M. R. & Monkman, A. P. Using guest–host interactions to optimize the efficiency of TADF OLEDs. J. Phys. Chem. Lett. 7, 3341–3346 (2016).
Deng, C., Zhang, L., Wang, D., Tsuboi, T. & Zhang, Q. Exciton‐ and polaron‐induced reversible dipole reorientation in amorphous organic semiconductor films. Adv. Opt. Mater. 7, 1801644 (2019).
Méhes, G., Goushi, K., Potscavage, W. J. & Adachi, C. Influence of host matrix on thermally-activated delayed fluorescence: effects on emission lifetime, photoluminescence quantum yield, and device performance. Org. Electron. 15, 2027–2037 (2014).
Olivier, Y. et al. Nature of the singlet and triplet excitations mediating thermally activated delayed fluorescence. Phys. Rev. Mater. 1, 075602 (2017).
Yersin, H., Mataranga-Popa, L., Czerwieniec, R. & Dovbii, Y. Design of a new mechanism beyond thermally activated delayed fluorescence toward fourth generation organic light emitting diodes. Chem. Mater. 31, 6110–6116 (2019).
Hosokai, T. et al. Solvent-dependent investigation of carbazole benzonitrile derivatives: does the 3LE−1CT energy gap facilitate thermally activated delayed fluorescence? J. Photonics Energy 8, 032102 (2018).
Han, C., Zhang, Z., Ding, D. & Xu, H. Dipole–dipole interaction management for efficient blue thermally activated delayed fluorescence diodes. Chem 4, 2154–2167 (2018).
Parusel, A. Semiempirical studies of solvent effects on the intramolecular charge transfer of the fluorescence probe PRODAN. J. Chem. Soc. Faraday Trans. 94, 2923–2927 (1998).
Northey, T., Stacey, J. & Penfold, T. J. The role of solid state solvation on the charge transfer state of a thermally activated delayed fluorescence emitter. J. Mater. Chem. C 5, 11001–11009 (2017).
Cui, L.-S. et al. Fast spin–flip enables efficient and stable organic electroluminescence from charge-transfer states. Nat. Photon. 14, 636–642 (2020).
Tang, X. et al. Highly efficient luminescence from space-confined charge-transfer emitters. Nat. Mater. 19, 1332–1338 (2020).
Kuang, Z. et al. Conformational relaxation and thermally activated delayed fluorescence in anthraquinone-based intramolecular charge-transfer compound. J. Phys. Chem. C 122, 3727–3737 (2018).
Delor, M. et al. Resolving and controlling photoinduced ultrafast solvation in the solid state. J. Phys. Chem. Lett. 8, 4183–4190 (2017).
Cotts, B. L. et al. Tuning thermally activated delayed fluorescence emitter photophysics through solvation in the solid state. ACS Energy Lett. 2, 1526–1533 (2017).
Wang, H. et al. Novel thermally activated delayed fluorescence materials–thioxanthone derivatives and their applications for highly efficient OLEDs. Adv. Mater. 26, 5198–5204 (2014).
Kaji, H. et al. Purely organic electroluminescent material realizing 100% conversion from electricity to light. Nat. Commun. 6, 8476 (2015).
Noguchi, Y. et al. Charge accumulation at organic semiconductor interfaces due to a permanent dipole moment and its orientational order in bilayer devices. J. Appl. Phys. 111, 114508 (2012).
Ritzoulis, G., Papadopoulos, N. & Jannakoudakis, D. Densities, viscosities, and dielectric constants of acetonitrile + toluene at 15, 25, and 35 °C. J. Chem. Eng. Data 31, 146–148 (1986).
Yu, S., Hing, P. & Hu, X. Dielectric properties of polystyrene–aluminum-nitride composites. J. Appl. Phys. 88, 398–404 (2000).
Skuodis, E. et al. Aggregation, thermal annealing, and hosting effects on performances of an acridan-based TADF emitter. Org. Electron. 63, 29–40 (2018).
Takeuchi, S. et al. Spectroscopic tracking of structural evolution in ultrafast stilbene photoisomerization. Science 322, 1073–1077 (2008).
Iwamura, M., Watanabe, H., Ishii, K., Takeuchi, S. & Tahara, T. Coherent nuclear dynamics in ultrafast photoinduced structural change of bis(diimine)copper(I) complex. J. Am. Chem. Soc. 133, 7728–7736 (2011).
Iwamura, M., Takeuchi, S. & Tahara, T. Ultrafast excited-state dynamics of copper(I) complexes. Acc. Chem. Res. 48, 782–791 (2015).
Karunakaran, V. & Das, S. Direct observation of cascade of photoinduced ultrafast intramolecular charge transfer dynamics in diphenyl acetylene derivatives: via solvation and intramolecular relaxation. J. Phys. Chem. B 120, 7016–7023 (2016).
Choi, J., Ahn, D.-S., Oang, K. Y., Cho, D. W. & Ihee, H. Charge transfer-induced torsional dynamics in the excited state of 2,6-bis(diphenylamino)anthraquinone. J. Phys. Chem. C 121, 24317–24323 (2017).
Petrozza, A., Laquai, F., Howard, I. A., Kim, J.-S. & Friend, R. H. Dielectric switching of the nature of excited singlet state in a donor-acceptor-type polyfluorene copolymer. Phys. Rev. B 81, 205421 (2010).
Hosokai, T. et al. Evidence and mechanism of efficient thermally activated delayed fluorescence promoted by delocalized excited states. Sci. Adv. 3, e1603282 (2017).
Liebel, M. & Kukura, P. Broad-band impulsive vibrational spectroscopy of excited electronic states in the time domain. J. Phys. Chem. Lett. 4, 1358–1364 (2013).
Sasaki, S., Drummen, G. P. C. & Konishi, G. I. Recent advances in twisted intramolecular charge transfer (TICT) fluorescence and related phenomena in materials chemistry. J. Mater. Chem. C 4, 2731–2743 (2016).
Santos, P. L. et al. Engineering the singlet–triplet energy splitting in a TADF molecule. J. Mater. Chem. C 4, 3815–3824 (2016).
Aizawa, N., Harabuchi, Y., Maeda, S. & Pu, Y.-J. Kinetic prediction of reverse intersystem crossing in organic donor–acceptor molecules. Nat. Commun. 11, 3909 (2020).
Niu, Y., Peng, Q. & Shuai, Z. Promoting-mode free formalism for excited state radiationless decay process with Duschinsky rotation effect. Sci. China Ser. B 51, 1153–1158 (2008).
Warshel, A. & Parson, W. W. Computer simulations of electron-transfer reactions in solution and in photosynthetic reaction centers. Annu. Rev. Phys. Chem. 42, 279–309 (1991).
Samanta, P. K., Kim, D., Coropceanu, V. & Brédas, J.-L. Up-conversion intersystem crossing rates in organic emitters for thermally activated delayed fluorescence: impact of the nature of singlet vs triplet excited states. J. Am. Chem. Soc. 139, 4042–4051 (2017).
de Mello, J. C., Wittmann, H. F. & Friend, R. H. An improved experimental determination of external photoluminescence quantum efficiency. Adv. Mater. 9, 230–232 (1997).
Grimme, S., Brandenburg, J. G., Bannwarth, C. & Hansen, A. Consistent structures and interactions by density functional theory with small atomic orbital basis sets. J. Chem. Phys. 143, 054107 (2015).
Neese, F. Software update: the ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 8, e1327 (2018).
Dhali, R., Phan Huu, D. K. A., Terenziani, F., Sissa, C. & Painelli, A. Thermally activated delayed fluorescence: a critical assessment of environmental effects on the singlet–triplet energy gap. J. Chem. Phys. 154, 134112 (2021).
Schmid, N. et al. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 40, 843–856 (2011).
Berendsen, H. J. C., van der Spoel, D. & van Drunen, R. GROMACS: a message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 91, 43–56 (1995).
Acknowledgements
A.J.G. and R.H.F. acknowledge support from the Simons Foundation (grant no. 601946) and the Engineering and Physical Sciences Research Council (EPSRC) (EP/M01083X/1 and EP/M005143/1). This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (R.H.F., grant agreement no. 670405; A.R., grant agreement no. 758826). A.R. thanks the Winton Programme for the Physics of Sustainability for funding. A.P., Y.O. and D.B. were supported by the European Union’s Horizon 2020 research and innovation programme under Marie Sklodowska Curie Grant agreement 748042 (MILORD project). Computational resources in Mons were provided by the Consortium des Équipements de Calcul Intensif, funded by the Fonds de la Recherche Scientifiques de Belgique (FNRS) under grant no. 2.5020.11, as well as by the Tier-1 supercomputer of the Fedération Wallonie-Bruxelles, infrastructure funded by the Walloon Region under grant agreement no. 1117545. D.B. is a FNRS Research Director. R.P. acknowledges financial support from an EPSRC Doctoral Prize Fellowship. A.J.S. acknowledges the Royal Society Te Apārangi and the Cambridge Commonwealth European and International Trust for their financial support. Y.O. acknowledges funding by the FNRS under grant no. F.4534.21 (MIS-IMAGINE). L.-S.C. acknowledges funding from the University of Science and Technology of China (USTC) Research Funds of the Double First-Class Initiative and the National Natural Science Foundation of China (grant no. 52103242), and this work was partially carried out at the USTC Center for Micro and Nanoscale Research and Fabrication. S.F. is grateful for support from an EPSRC Doctoral Prize Fellowship and the Winton Programme for the for the Physics of Sustainability. We thank C. Schnedermann for useful discussions.
Author information
Authors and Affiliations
Contributions
A.J.G, R.H.F. and D.B. conceived the work. A.J.G. performed the TA measurements. A.J.G., R.P. and A.J.S. carried out the IVS measurements. S.F. performed the transient grating PL measurements. E.W.E., T.H.T. and B.H.D. carried out the time-resolved PL measurements. E.W.E. measured the PL quantum efficiency. T.H.T. performed the Raman spectroscopy. A.J.G., E.W.E and L.-S.C. fabricated the samples used in the work. A.P. carried out the quantum-chemical calculations. A.M.A., G.D.S, Y.O. and D.B. discussed the calculation results. A.R., R.H.F. and D.B. supervised their group members involved in the project. A.J.G., A.P., G.D.S., R.H.F. and D.B. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Takuya Hosokai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–35 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Gillett, A.J., Pershin, A., Pandya, R. et al. Dielectric control of reverse intersystem crossing in thermally activated delayed fluorescence emitters. Nat. Mater. 21, 1150–1157 (2022). https://doi.org/10.1038/s41563-022-01321-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01321-2
This article is cited by
-
A figure of merit for efficiency roll-off in TADF-based organic LEDs
Nature (2024)
-
Confining donor conformation distributions for efficient thermally activated delayed fluorescence with fast spin-flipping
Nature Communications (2023)
-
A direct observation of up-converted room-temperature phosphorescence in an anti-Kasha dopant-matrix system
Nature Communications (2023)
-
Advanced charge transfer technology for highly efficient and long-lived TADF-type organic afterglow with near-infrared light-excitable property
Science China Chemistry (2023)