Abstract
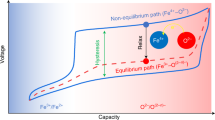
Reversible anionic redox reactions represent a transformational change for creating advanced high-energy-density positive-electrode materials for lithium-ion batteries. The activation mechanism of these reactions is frequently linked to ligand-to-metal charge transfer (LMCT) processes, which have not been fully validated experimentally due to the lack of suitable model materials. Here we show that the activation of anionic redox in cation-disordered rock-salt Li1.17Ti0.58Ni0.25O2 involves a long-lived intermediate Ni3+/4+ species, which can fully evolve to Ni2+ during relaxation. Combining electrochemical analysis and spectroscopic techniques, we quantitatively identified that the reduction of this Ni3+/4+ species goes through a dynamic LMCT process (Ni3+/4+–O2− → Ni2+–On−). Our findings provide experimental validation of previous theoretical hypotheses and help to rationalize several peculiarities associated with anionic redox, such as cationic–anionic redox inversion and voltage hysteresis. This work also provides additional guidance for designing high-capacity electrodes by screening appropriate cationic species for mediating LMCT.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this article and its Supplementary Information will be made available upon reasonable request to the authors. Source data are provided with this paper.
References
Rozier, P. & Tarascon, J. M. Review—Li-rich layered oxide cathodes for next-generation li-ion batteries: chances and challenges. J. Electrochem. Soc. 162, A2490 (2015).
Yabuuchi, N. Material design concept of lithium-excess electrode materials with rocksalt-related structures for rechargeable non-aqueous batteries. Chem. Rec. 19, 690 (2018).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Assat, G. & Tarascon, J.-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy 3, 373–386 (2018).
Li, B. & Xia, D. Anionic redox in rechargeable lithium batteries. Adv. Mater. 29, 1701054 (2017).
Li, M. et al. Cationic and anionic redox in lithium-ion based batteries. Chem. Soc. Rev. 49, 1688–1705 (2020).
Assat, G., Delacourt, C., Corte, D. A. D. & Tarascon, J.-M. Editors’ choice—Practical assessment of anionic redox in Li-rich layered oxide cathodes: a mixed blessing for high energy Li-ion batteries. J. Electrochem. Soc. 163, A2965–A2976 (2016).
Seo, D. H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Abakumov, A. M., Fedotov, S. S., Antipov, E. V. & Tarascon, J. M. Solid state chemistry for developing better metal-ion batteries. Nat. Commun. 11, 4976 (2020).
Ben Yahia, M., Vergnet, J., Saubanere, M. & Doublet, M. L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 18, 496–502 (2019).
Li, B. et al. Thermodynamic activation of charge transfer in anionic redox process for Li-ion batteries. Adv. Funct. Mater. 28, 1704864 (2018).
Saha, S. et al. Exploring the bottlenecks of anionic redox in Li-rich layered sulfides. Nat. Energy 4, 977–987 (2019).
Flamary-Mespoulie, F. et al. Lithium-rich layered titanium sulfides: cobalt- and nickel-free high capacity cathode materials for lithium-ion batteries. Energy Storage Mater. 26, 213–222 (2020).
Leube, B. T. et al. Activation of anionic redox in d(0) transition metal chalcogenides by anion doping. Nat. Commun. 12, 5485 (2021).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4-based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Hong, J. et al. Metal-oxygen decoordination stabilizes anion redox in Li-rich oxides. Nat. Mater. 18, 256–265 (2019).
Radin, M. D., Vinckeviciute, J., Seshadri, R. & Vander Ven, A. Manganese oxidation as the origin of the anomalous capacity of Mn-containing Li-excess cathode materials. Nat. Energy 4, 639–646 (2019).
Li, B. et al. Correlating ligand-to-metal charge transfer with voltage hysteresis in a Li-rich rock-salt compound exhibiting anionic redox. Nat. Chem. 13, 1070–1080 (2021).
De Ridder, R., van Tendeloo, G. & Amelinckx, S. A cluster model for the transition from the short-range order to the long-range order state in f.c.c. based binary systems and its study by means of electron diffraction. Acta Crystallogr. A 32, 216–224 (1976).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014).
Jacquet, Q. et al. Charge transfer band gap as an indicator of hysteresis in Li-disordered rock salt cathodes for Li-ion batteries. J. Am. Chem. Soc. 141, 11452–11464 (2019).
Farges, F. Coordination of Ti4+ in silicate glasses: a high-resolution XANES spectroscopy study at the Ti K-edge. Am. Mineral. 82, 36–43 (1997).
Achkar, A. J., Regier, T. Z., Monkman, E. J., Shen, K. M. & Hawthorn, D. G. Determination of total X-ray absorption coefficient using non-resonant X-ray emission. Sci. Rep. 1, 182 (2011).
Qiao, R. et al. Direct experimental probe of the Ni(II)/Ni(III)/Ni(IV) redox evolution in LiNi0.5Mn1.5O4 electrodes. J. Phys. Chem. C 119, 27228–27233 (2015).
Yoon, W.-S., Chung, K. Y., McBreen, J., Fischer, D. A. & Yang, X.-Q. Changes in electronic structure of the electrochemically Li-ion deintercalated LiNiO2 system investigated by soft X-ray absorption spectroscopy. J. Power Sources 163, 234–237 (2006).
Croy, J. R. et al. Examining hysteresis in composite xLi2MnO3·(1−x)LiMO2 cathode structures. J. Phys. Chem. C 117, 6525–6536 (2013).
Sawatzky, G. A. & Allen, J. W. Magnitude and origin of the band gap in NiO. Phys. Rev. Lett. 53, 2339–2342 (1984).
Assat, G., Iadecola, A., Delacourt, C., Dedryvère, R. & Tarascon, J.-M. Decoupling cationic–anionic redox processes in a model Li-rich cathode via operando X-ray absorption spectroscopy. Chem. Mater. 29, 9714–9724 (2017).
Marcus, R. A. Electron transfer reactions in chemistry. Theory and experiment. Rev. Mod. Phys. 65, 599–610 (1993).
Kim, M. G. et al. Ni and oxygen K-edge XAS investigation into the chemical bonding for lithiation of LiyNi1−xAlxO2 cathode material. Electrochim. Acta 50, 501–504 (2004).
Kleiner, K. et al. On the origin of reversible and irreversible reactions in LiNixCo(1−x)/2Mn(1−x)/2O2. J. Electrochem. Soc. https://doi.org/10.1149/1945-7111/ac3c21 (2021).
Zhuo, Z. et al. Distinct oxygen redox activities in Li2MO3 (M = Mn, Ru, Ir). ACS Energy Lett. 6, 3417–3424 (2021).
Bianchini, M. et al. From LiNiO2 to Li2NiO3: synthesis, structures and electrochemical mechanisms in Li-rich nickel oxides. Chem. Mater. 32, 9211–9227 (2020).
Pearce, P. E. et al. Evidence for anionic redox activity in a tridimensional-ordered Li-rich positive electrode β-Li2IrO3. Nat. Mater. 16, 580–586 (2017).
Assat, G. et al. Fundamental interplay between anionic/cationic redox governing the kinetics and thermodynamics of lithium-rich cathodes. Nat. Commun. 8, 2219 (2017).
Yabuuchi, N. et al. Origin of stabilization and destabilization in solid-state redox reaction of oxide ions for lithium-ion batteries. Nat. Commun. 7, 13814 (2016).
Taylor, Z. N. et al. Stabilization of O–O bonds by d(0) cations in Li4+xNi1−xWO6 (0 ≤ x ≤ 0.25) rock salt oxides as the origin of large voltage hysteresis. J. Am. Chem. Soc. 141, 7333–7346 (2019).
Yabuuchi, N., Tahara, Y., Komaba, S., Kitada, S. & Kajiya, Y. Synthesis and electrochemical properties of Li4MoO5–NiO binary system as positive electrode materials for rechargeable lithium batteries. Chem. Mater. 28, 416–419 (2016).
Leube, B. T. et al. Layered sodium titanium trichalcogenide Na2TiCh3 framework (Ch = S, Se): a rich crystal and electrochemical chemistry. Chem. Mater. https://doi.org/10.1021/acs.chemmater.1c04374 (2022).
Barbara, P. F., Meyer, T. J. & Ratner, M. A. Contemporary issues in electron transfer research. J. Phys. Chem. C 100, 13148–13168 (1996).
Wegeberg, C. & Wenger, O. S. Luminescent first-row transition metal complexes. JACS Au 1, 1860–1876 (2021).
Chabera, P. et al. A low-spin Fe(III) complex with 100-ps ligand-to-metal charge transfer photoluminescence. Nature 543, 695–699 (2017).
Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55–69 (1993).
Zhang, L. et al. Unraveling gas evolution in sodium batteries by online electrochemical mass spectrometry. Energy Storage Mater. 42, 12–21 (2021).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Rueff, J. P., Rault, J. E., Ablett, J. M., Utsumi, Y. & Céolin, D. HAXPES for materials science at the GALAXIES beamline. Synchrotron Radiat. News 31, 4–9 (2018).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Wang, L., Maxisch, T. & Ceder, G. Oxidation energies of transition metal oxides within the GGA + U framework. Phys. Rev. B 73,195107 (2006).
Acknowledgements
This research used resources of the Advanced Photon Source, a US Department of Energy (DOE) Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under contract number DE-AC02-06CH11357. We are grateful to J. Freeland, T. Wu and G. Sterbinsky for their help during mail-in XAS measurements at the Advanced Photon Source. J.C. and I.R. acknowledge support from the National Science Foundation, under grant number DMR-1809372. K.K. acknowledges support from the National Science Foundation, under grant number CBET-1800357. A.M.A. and A.V.M. are grateful to the Russian Science Foundation for financial support (grant 20-13-00233). Access to TEM facilities was granted by the Advance Imaging Core Facility of Skoltech. HAXPES experiments (proposal no. 99210184) were performed on the GALAXIES beamline at the SOLEIL Synchrotron, France. The Ni K-edge XAS was collected on the ROCK beamline at the SOLEIL Synchrotron through a rapid access for urgent need. We are grateful to J. Sottmann and J.-P. Rueff for their assistance during the HAXPES experiments. J.-M.T and B.L. acknowledge funding from the European Research Council (ERC) (FP/2014)/ERC Grant-Project 670116-ARPEMA.
Author information
Authors and Affiliations
Contributions
B.L. and J.-M.T. conceived the idea and designed the experiments. B.L. carried out the synthesis, structural characterization, electrochemical analysis and DFT calculations. R.D. collected and analysed the HAXPES data. K.K., I.R. and J.C. collected the XAS data and carried out the analysis. A.V.M., O.V.E. and A.M.A. performed TEM experiments and did the analysis. L.Z. performed the OEMS experiments and data analysis. S.B. collected the Ni K-edge XAS data during relaxation. T.K. did the protocol and assembling of ASSB cells. B.L. and J.-M.T. wrote the manuscript with the contributions from all the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Kisuk Kang and Zhaoxiang Wang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–34, Notes I–III and Table 1.
Source data
Source Data Fig. 1
Statistical source data
Source Data Fig. 2
Statistical source data
Source Data Fig. 4
Statistical source data
Source Data Fig. 6
Statistical source data
Rights and permissions
About this article
Cite this article
Li, B., Kumar, K., Roy, I. et al. Capturing dynamic ligand-to-metal charge transfer with a long-lived cationic intermediate for anionic redox. Nat. Mater. 21, 1165–1174 (2022). https://doi.org/10.1038/s41563-022-01278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01278-2
This article is cited by
-
An air-stable single-crystal layered oxide cathode based on multifunctional structural modulation for high-energy-density sodium-ion batteries
Science China Chemistry (2024)
-
Decoupling the roles of Ni and Co in anionic redox activity of Li-rich NMC cathodes
Nature Materials (2023)
-
Tuning oxygen redox via transition-metal cation species
Science China Chemistry (2023)
-
Building Better Full Manganese-Based Cathode Materials for Next-Generation Lithium-Ion Batteries
Electrochemical Energy Reviews (2023)
-
Real-space measurement of orbital electron populations for Li1-xCoO2
Nature Communications (2022)