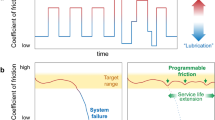
Abstract
Room-temperature ionic liquids and their mixtures with organic solvents as lubricants open a route to control lubricity at the nanoscale via electrical polarization of the sliding surfaces. Electronanotribology is an emerging field that has a potential to realize in situ control of friction—that is, turning the friction on and off on demand. However, fulfilling its promise needs more research. Here we provide an overview of this emerging research area, from its birth to the current state, reviewing the main achievements in non-equilibrium molecular dynamics simulations and experiments using atomic force microscopes and surface force apparatus. We also present a discussion of the challenges that need to be solved for future applications of electrotunable friction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Holmberg, K. & Erdemir, A. Influence of tribology on global energy consumption, costs and emissions. Friction 5, 263–284 (2017).
Jost, P. Lubrication (Tribology)—A Report on the Present Position and Industry’s Needs (Department of Education and Science, HM Stationery Office, 1966)
Amontons, G. De la resistance cause’e dans les machines (About resistance and force in machines). Mem. Aced. R. A. 257–282 (1699).
Hutchings, I. M. Leonardo da Vinci’s Studies of Friction. Wear 360, 51–66 (2016).
Coulomb, C. A. Sur une application des regles de maximis & minimis a quelques problemes de statique, relatifs a L’architecture. Mem. Math. Phys. 7, 343–382 (1773).
Chaston, J. C. Wear resistance of gold alloys for coinage. Gold Bull. 7, 108–112 (1974).
Bowden, F. P. & Tabor, D. The Friction and Lubrication of Solids (Oxford Univ. Press, 1950).
Urbakh, M., Klafter, J., Gourdon, D. & Israelachvili, J. The nonlinear nature of friction. Nature 430, 525–528 (2004).
Szlufarska, I., Chandross, M. & Carpick, R. W. Recent advances in single-asperity nanotribology. J. Phys. D 41, 123001 (2008).
Vanossi, A., Manini, N., Urbakh, M., Zaperri, S. & Tosatti, E. Modeling friction: from nanoscale to mesoscale. Rev. Mod. Phys. 85, 529–551 (2013).
Rapoport, L., Fleischer, N. & Tenne, R. Fullerene-like WS2 nanoparticles: superior lubricants for harsh conditions. Adv. Mater. 15, 651–655 (2003).
Palacio, M. & Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 40, 247–268 (2010).
Raviv, U. & Klein, J. Fluidity of bound hydration layers. Science 297, 1540–1543 (2002).
Vanossi, A., Bechinger, C. & Urbakh, M. Structural lubricity in soft and hard matter systems. Nat. Commun. 11, 4657 (2020).
Rozman, M. G., Urbakh, M. & Klafter, J. Controlling chaotic frictional forces. Phys. Rev. E 57, 7340–7343 (1998).
Socoliuc, A. et al. Atomic-scale control of friction by actuation of nanometersized contacts. Science 313, 207–210 (2006).
Sasak, M., Xu, Y. & Goto, M. Control of friction force by light observed by friction force microscopy in a vacuum. Appl. Phys. Express 10, 015201 (2017).
Spikes, H. A. Triboelectrochemistry: influence of applied electrical potentials on friction and wear of lubricated contacts. Tribol. Lett. 68, 90 (2020).
Krim, J. Controlling friction with external electric or magnetic fields: 25 examples. Front. Mech. Eng. 5, 22 (2019).
Hausen, F., Gosvami, N. N. & Bennewitz, R. Anion adsorption and atomic friction on Au(111). Electrochim. Acta 56, 10694–10700 (2011).
Sweeney, J. et al. Control of nanoscale friction on gold in ionic liquid by a potential dependent ionic lubricant layer. Phys. Rev. Lett. 109, 155502 (2012).
Li, H., Wood, R. J., Rutland, M. W. & Atkin, R. An ionic liquid lubricant enables superlubricity to be ‘switched on’ in situ using an electrical potential. Chem. Commun. 50, 4368–4370 (2014).
Strelcov, E. et al. Nanoscale lubrication of ionic surfaces controlled via a strong electric field. Sci. Rep. 5, 8049 (2015).
Krämer, G., Hausen, F. & Bennewitz, R. Dynamic shear force microscopy of confined liquids at a gold electrode. Faraday Discuss. 199, 299–309 (2017).
Israelachvili, J. Intermolecular and Surface Forces 3rd edn (Academic, 2011).
Perkin, S., Albrecht, T. & Klein, J. Layering and shear properties of an ionic liquid, 1-ethyl-3-methylimidazolium ethylsulfate, confined to nano-films between mica surfaces. Phys. Chem. Chem. Phys. 12, 1243–1247 (2010).
Fre´chette, J. & Vanderlick, T. K. Double layer forces over large potential ranges as measured in an electrochemical surface forces apparatus. Langmuir 17, 7620–7627 (2001).
Valtiner, M. et al. The electrochemical surface forces apparatus: the effect of surface roughness, electrostatic surface potentials, and anodic oxide growth on interaction forces, and friction between dissimilar surfaces in aqueous solutions. Langmuir 28, 13080–13093 (2012).
Britton, J. et al. A graphene surface force balance. Langmuir 30, 11485–11492 (2014).
Tivony, R. & Klein, J. Modifying surface forces through control of surface potentials. Faraday Discuss. 199, 261–277 (2017).
Perez Martinez, C. S. & Perkin, S. Surface forces generated by the action of electric fields across liquid films. Soft Matter 15, 4255–4265 (2019).
Tivony, R., Yaakov, D. B., Silbert, G. & Klein, J. Direct observation of confinement-induced charge inversion at a metal surface. Langmuir 31, 12845–12849 (2015).
Hallett, J. P. & Welton, T. Room-temperature ionic liquids: solvents for synthesis and catalysis. Chem. Rev. 111, 3508–3576 (2011).
Fedorov, M. V. & Kornyshev, A. A. Ionic liquids at electrified interfaces. Chem. Rev. 114, 2978–3036 (2014).
Hayes, R., Warr, G. G. & Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 115, 6357–6426 (2015).
Miami, I., Inada, T., Sasaki, R. & Nanao, H. Tribo-chemistry of phosphonium-derived ionic liquids. Tribol. Lett. 40, 225–235 (2010).
Wang, H., Lu, Q., Ye, C., Liu, W. & Cui, Z. Friction and wear behaviors of ionic liquid of alkylimidazolium hexaflurophophates as lubricants for steel/steel contact. Wear 256, 44–48 (2004).
Somers, A. E., Howlett, P. C., MacFarlane, D. R. & Forsyth, M. S. Review of ionic liquid lubricants. Lubricants 1, 3–21 (2013).
Bazant, M. Z., Storey, B. D. & Kornyshev, A. A. Double layer in ionic liquids: overscreening versus crowding. Phys. Rev. Lett. 106, 046102 (2011).
Smith, A. M., Lovelock, K. R. J., Gosvami, N. N., Welton, T. & Perkin, S. Quantized friction across ionic liquid thin films. Phys. Chem. Chem. Phys. 15, 15317–15320 (2013).
Comtet, J. et al. Nanoscale capillary freezing of ionic liquids confined between metallic interfaces and the role of electronic screening. Nat. Mater. 16, 634–639 (2017).
Zhang, Y., Rutland, M. W., Luo, J., Atkin, R. & Li, H. Potential-dependent superlubricity of ionic liquids on a graphite surface. J. Phys. Chem. C 125, 3940–3947 (2021).
Hoth, J., Hausen, F., Müser, M. H. & Bennewitz, R. Force microscopy of layering and friction in an ionic liquid. J. Phys. Condens. Matter 26, 284110 (2014).
Jurado, L. A. et al. Irreversible structural change of a dry ionic liquid under nanoconfinement. Phys. Chem. Chem. Phys. 17, 13613–13624 (2015).
Black, J. M. et al. Fundamental aspects of electric double layer force-distance measurements at liquid-solid interfaces using atomic force microscopy. Sci. Rep. 6, 32389 (2016).
Ebeling, D., Bradler, S., Roling, B. & Schirmeisen, A. 3‑dimensional structure of a prototypical ionic liquid−solid interface: ionic crystal-like behavior induced by molecule−substrate interactions. J. Phys. Chem. C 120, 11947–11955 (2016).
Goodwin, Z. A. H., Eikerling, M., Loewen, H. & Kornyshev, A. A. Theory of microstructured polymer electrolyte artificial muscles. Smart Mater. Struct. 27, 075056 (2018).
Kornyshev, A. A. et al. Ultra-low voltage electrowetting. J. Phys. Chem. C 114, 14885–14890 (2010).
Cai, M., Yu, Q., Liu, W. & Zhou, F. Ionic liquid lubricants: when chemistry meets tribology. Chem. Soc. Rev. 49, 7753–7818 (2020).
Drummond, C. Electric-field-induced friction reduction and control. Phys. Rev. Lett. 109, 154302 (2012).
Tivony, R., Safran, S., Pincus, P., Silbert, G. & Klein, J. Charging dynamics of an individual nanopore. Nat. Commun. 9, 4203 (2018).
van Engers, C. D., Balabajew, M., Southam, A. & Perkin, S. A 3-mirror surface force balance for the investigation of fluids confined to nanoscale films between two ultra-smooth polarizable electrodes. Rev. Sci. Instrum. 89, 123901 (2018).
Black, J. M. et al. Bias-dependent molecular-level structure of electrical double layer in ionic liquid on graphite. Nano Lett. 13, 5954–596 (2013).
Smith, A. M., Parkes, M. A. & Perkin, S. Molecular friction mechanisms across nanofilms of a bilayer-forming ionic liquid. J. Phys. Chem. Lett. 5, 4032–4037 (2014).
Espinosa-Marzal, R. M., Arcifa, A., Rossi, A. & Spencer, N. D. Microslips to ‘avalanches’ in confined, molecular layers of ionic liquids. J. Phys. Chem. Lett. 5, 179–184 (2014).
Perez-Martinez, C. & Perkin, S. Interfacial structure and boundary lubrication of a dicationic ionic liquid. Langmuir 35, 15444–15450 (2019).
Fajardo, O. Y., Bresme, F., Kornyshev, A. A. & Urbakh, M. Water in ionic liquid lubricants: friend and foe. ACS Nano 11, 6825–6831 (2017).
Capozza, R., Vanossi, A., Benassi, A. & Tosatti, E. Squeezout phenomena and boundary layer formation of a model ionic liquid under confinement and charging. J. Chem. Phys. 142, 064707 (2015).
Di Lecce, S., Kornyshev, A. A., Urbakh, M. & Bresme, F. Electrotunable lubrication with ionic liquids: the effects of cation chain length and substrate polarity. ACS Appl. Mater. Interf. 12, 4105–4113 (2020).
Fajardo, O. Y., Bresme, F., Kornyshev, A. A. & Urbakh, M. Electrotunable lubricity with ionic liquid nanoscale films. Sci. Rep. 5, 7698 (2015).
Canova, F. F., Matsubara, H., Mizukami, M., Kurihara, K. & Shluger, A. L. Shear dynamics of nanoconfined ionic liquids. Phys. Chem. Chem. Phys. 16, 8247–8256 (2014).
Merlet, C. et al. Simulating supercapacitors: can we model electrodes as constant charge surfaces. J. Phys. Chem. Lett. 4, 264–268 (2013).
Fajardo, O. Y., Bresme, F., Kornyshev, A. A. & Urbakh, M. Electrotunable friction with ionic liquid lubricants: how important is the molecular structure of the ions? J. Phys. Chem. Lett. 6, 3998–4004 (2015).
Pivnic, K., Bresme, F., Kornyshev, A. A. & Urbakh, M. Structural forces in mixtures of ionic liquids with organic solvents. Langmuir 35, 15410–15420 (2019).
Yan, Z. et al. Two dimensional ordering of ionic liquids confined by layered silicate plates via molecular dynamics simulation. J. Phys. Chem. C 119, 19244–19252 (2015).
Begic, S., Jonsson, E., Chen, F. & Forsyth, M. Molecular dynamics simulations of pyrrolidinium and imidazoium ionic liquids at graphene interfaces. Phys. Chem. Chem. Phys. 19, 30010 (2017).
Dašić, M., Stankovic, I. & Gkagkas, K. Molecular dynamics investigation of the influence of the shape of the cation on the structure and lubrication properties of ionic liquids. Phys. Chem. Chem. Phys. 21, 4375–4386 (2019).
Capozza, R., Benassi, A., Vanossi, A. & Tosatti, E. Electrical charging effects on the sliding friction of a model nano-confined ionic liquid. J. Chem. Phys. 143, 144703 (2015).
Kramer, G. & Bennewitz, R. Molecular rheology of a nanometer-confined ionic liquid. J. Phys. Chem. C 123, 28284–28290 (2019).
Zhou, H. et al. Nanoscale perturbations of room temperature ionic liquid structure at charged and uncharged interfaces. ACS Nano 6, 9818–9827 (2012).
Velpula, G. et al. Graphene meets ionic liquids: Fermi level engineering via electrostatic forces. ACS Nano 13, 3512–3521 (2019).
Zhang, F., Fang, C. & Qiao, R. Effects of water on mica−ionic liquid interfaces. J. Phys. Chem. C 122, 9035–9045 (2018).
Di Lecce, S., Kornyshev, A. A., Urbakh, M. & Bresme, F. Lateral ordering in nanoscale ionic liquid films between charged surfaces enhances lubricity. ACS Nano 14, 13256–13267 (2020).
David, A., Fajardo, O. Y., Kornyshev, A. A., Urbakh, M. & Bresme, F. Electrotunable lubricity with ionic liquids: the influence of nanoscale roughness. Faraday Discuss. 199, 279 (2017).
Richter, Ł. et al. Ions in an AC electric field: strong long-range repulsion between oppositely charged surfaces. Phys. Rev. Lett. 125, 056001 (2020).
Perez-Martinez, C. S., Groves, T. & Perkin, S. Controlling adhesion using AC electric fields across fluid films. J. Phys. Condens. Matter 33, 31LT02 (2021).
Balabajew, M., van Engers, C. D. & Perkin, S. Contact-free calibration of an asymmetric multi-layer interferometer for the surface force balance. Rev. Sci. Instrum. 88, 123903 (2017).
Glavatskhi, S. & Hoglund, E. Tribotronics-Towards active tribology. Tribol. Int. 41, 934–939 (2008).
Pam, S. & Zhang, Z. Fundamental theories and basic principles of triboelectric effect: a review. Friction 7, 2–17 (2019).
Coles, S. W., Smith, A. M., Fedorov, M. V., Hausen, F. & Perkin, S. Interfacial structure and structural forces in mixtures of ionic liquid with a polar solvent. Faraday Discuss. 206, 427 (2018).
Cooper, P. K., Li, H., Rutland, M. W., Webber, G. B. & Atkin, R. Tribotronic control of friction in oil-based lubricants with ionic liquid additives. Phys. Chem. Chem. Phys. 18, 23657 (2016).
Espinoza-Marzal, R. M., Arcifa, A., Rossi, A. & Spencer, N. D. Ionic liquids confined in hydrophobic nanocontacts: structure and lubricity in the presence of water. J. Phys. Chem. C 118, 6491–6503 (2014).
Watanabe, S. et al. Interfacial structuring of non-halogenated imidazolium ionic liquids at charged surfaces: effect of alkyl chain length. Phys. Chem. Chem. Phys. 22, 8450–8460 (2020).
Rollins, J. B., Fitchett, B. D. & Conboy, J. C. Structure and orientation of the imidazolium cation at the room-temperature ionic liquid/SiO2 interface measured by sum-frequency vibrational spectroscopy. J. Chem. Phys. B 111, 4990–4999 (2007).
Bresme, F., Lervik, A. & Armstrong, J. in Experimental Thermodynamics Volume X: Non-Equilibrium Thermodynamics with Applications (eds Bedeaux, D. et al.) 105 (IUPAC, 2016).
Roy, D. & Maroncelli, M. An improved four-site ionic liquid model. J. Phys. Chem. B 114, 12629–12631 (2010).
Fajardo, O. Y., Di Lecce, S. & Bresme, F. Molecular dynamics simulation of imidazolium CnMIM-BF4 ionic liquids using a coarse grained force-field. Phys. Chem. Chem. Phys. 22, 1682–1692 (2020).
Lopes, J. N. C., Deschamps, J. H. & Pádua, A. A. Modeling ionic liquids using a systematic all-atom force field. J. Phys. Chem. B 108, 2038–2047 (2004).
Yan, T., Burnham, C. J., Del Popolo, M. G. & Voth, G. A. Molecular dynamics simulation of ionic liquids: the effect of electronic polarizability. J. Phys. Chem. B 108, 12 (2004).
Nalam, P. C., Sheehan, A., Han, M. & Espinosa-Marzal, R. M. Effects of nanoscale roughness on the lubricious behavior of an ionic liquid. Adv. Mater. Interf. 7, 2000314 (2020).
Pivnic, K., Bresme, F., Kornyshev, A. A. & Urbakh, M. Electrotunable friction in diluted room temperature ionic liquids: implications for nanotribology. ACS Appl. Nano. Mat. 3, 10708–10719 (2020).
Seidl, C., Hormann, J. L. & Pastewka, L. Molecular Simulations of electrotunable lubrication: viscosity and wall slip in aqueous electrolytes. Tribol. Lett. 69, 22 (2021).
Ntim, S. & Sulpizi, M. Role of image charges in ionic liquid confined between metallic interfaces. Phys. Chem. Chem. Phys. 22, 10786–10791 (2020).
Liu, X. Z. et al. Dynamics of atomic stick-slip friction examined with atomic force microscopy and atomistic simulations at overlapping speeds. Rev. Lett. 114, 146102 (2015).
Ta, H. T. T. et al. Computational tribochemistry: a review from classical and quantum mechanical studies. J. Phys. Chem. C 125, 16875 (2021).
Feng, G., Jiang, X., Qiao, R. & Kornyshev, A. A. Water in ionic liquids at electrified interfaces: the anatomy of electrosorption. ACS Nano 8, 11685–11694 (2014).
Bi, S. et al. Minimizing the electrosorption of water from humid ionic liquids on electrodes. Nat. Commun. 9, 5222 (2018).
McEldrew, M., Goodwin, Z. A. H., Kornyshev, A. A. & Bazant, M. Z. Theory of the double layer in water-in-salt electrolytes. J. Phys. Chem. Lett. 9, 5840–5846 (2018).
Lertola, A. C., Wang, B. & Li, L. Understanding the friction of nanometer-thick fluorinated ionic liquids. Ind. Eng. Chem. Res. 57, 11681–11685 (2018).
Wang, J., Tian, Y., Zhao, Y. & Zhuo, K. A volumetric and viscosity study for the mixtures of 1-n-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with acetonitrile, dichloromethane, 2-butanone and N, N-dimethylformamide. Green Chem. 5, 618–622 (2003).
Li, S. et al. Enhanced performance of dicationic ionic liquid electrolytes by organic solvents. J. Phys. Condens. Matter 26, 284105 (2014).
Yang, X., Meng, Y. & Tian, Y. Effect of imidazolium ionic liquid additives on lubrication performance of propylene carbonate under different electrical potentials. Tribol. Lett. 56, 161–169 (2014).
Acknowledgements
F.B, A.A.K. and M.U. thank the Leverhulme Trust for the award of research grant number RPG-2016-223. M.U. acknowledges the financial support of the Israel Science Foundation under grant number 1141/18 and the binational programme of the National Science Foundation of China and Israel Science Foundation under grant number 3191/19. S.P. acknowledges support from the European Research Council under grant number 676861. We also thank R. Bennewitz for sharing high-resolution versions Figs. 2a and 3b.
Author information
Authors and Affiliations
Contributions
F.B., A.A.K., S.P. and M.U. conceived the idea of writing this Review, devised its general structure, designed the figures and contributed to the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Rob Atkin, Ali Erdemir and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bresme, F., Kornyshev, A.A., Perkin, S. et al. Electrotunable friction with ionic liquid lubricants. Nat. Mater. 21, 848–858 (2022). https://doi.org/10.1038/s41563-022-01273-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01273-7
This article is cited by
-
Boundary slip and lubrication mechanisms of organic friction modifiers with effect of surface moisture
Friction (2024)
-
Enhancing boundary friction and wear reduction through adsorption control in protic ionic liquid and carbon mixtures
Journal of Materials Science (2024)
-
Liquid-like polymer lubricating surfaces: Mechanism and applications
Nano Research (2024)
-
Structure and Reactivity of the Ionic Liquid [C1C1Im][Tf2N] on Cu(111)
Topics in Catalysis (2023)
-
Observation of enhanced nanoscale creep flow of crystalline metals enabled by controlling surface wettability
Nature Communications (2022)