Abstract
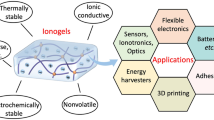
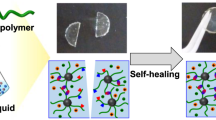
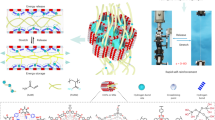
Ionogels are compelling materials for technological devices due to their excellent ionic conductivity, thermal and electrochemical stability, and non-volatility. However, most existing ionogels suffer from low strength and toughness. Here, we report a simple one-step method to achieve ultra-tough and stretchable ionogels by randomly copolymerizing two common monomers with distinct solubility of the corresponding polymers in an ionic liquid. Copolymerization of acrylamide and acrylic acid in 1-ethyl-3-methylimidazolium ethyl sulfate results in a macroscopically homogeneous covalent network with in situ phase separation: a polymer-rich phase with hydrogen bonds that dissipate energy and toughen the ionogel; and an elastic solvent-rich phase that enables for large strain. These ionogels have high fracture strength (12.6 MPa), fracture energy (~24 kJ m−2) and Young’s modulus (46.5 MPa), while being highly stretchable (~600% strain) and having self-healing and shape-memory properties. This concept can be applied to other monomers and ionic liquids, offering a promising way to tune ionogel microstructure and properties in situ during one-step polymerization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data generated or analysed during this study are provided as Source Data or included in the Supplementary Information. Further data are available from the corresponding authors on request. Further details on the methods are available in the Supplementary Information.
Reference
Lodge, T. P. & Ueki, T. Mechanically tunable, readily processable ion gels by self-assembly of block copolymers in ionic liquids. Acc. Chem. Res. 49, 2107–2114 (2016).
Ding, Y. et al. Preparation of high‐performance ionogels with excellent transparency, good mechanical strength, and high conductivity. Adv. Mater. 29, 1704253 (2017).
Zhou, B. et al. Flexible, self-healing, and fire-resistant polymer electrolytes fabricated via photopolymerization for all-solid-state lithium metal batteries. ACS Macro Lett. 9, 525–532 (2020).
Shi, L. et al. Highly stretchable and transparent ionic conductor with novel hydrophobicity and extreme-temperature tolerance. Research 2020, 2505619 (2020).
Tamate, R. et al. Self‐healing micellar ion gels based on multiple hydrogen bonding. Adv. Mater. 30, 1802792 (2018).
Kim, Y. M. & Moon, H. C. Ionoskins: nonvolatile, highly transparent, ultrastretchable ionic sensory platforms for wearable electronics. Adv. Funct. Mater. 30, 1907290 (2020).
Ren, Y. et al. Ionic liquid–based click-ionogels. Sci. Adv. 5, eaax0648 (2019).
Lu, B. et al. Pure PEDOT: PSS hydrogels. Nat. Commun. 10, 1043 (2019).
Xu, J. et al. Multi-scale ordering in highly stretchable polymer semiconducting films. Nat. Mater. 18, 594–601 (2019).
Liu, Y. et al. Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation. Nat. Biomed. Eng. 3, 58–68 (2019).
Ma, Y. et al. Flexible hybrid electronics for digital healthcare. Adv. Mater. 32, 1902062 (2020).
Kim, H. J., Chen, B., Suo, Z. & Hayward, R. C. Ionoelastomer junctions between polymer networks of fixed anions and cations. Science 367, 773–776 (2020).
Osada, I., de Vries, H., Scrosati, B. & Passerini, S. Ionic‐liquid‐based polymer electrolytes for battery applications. Angew. Chem. Int. Ed. Engl. 55, 500–513 (2016).
Chen, N., Zhang, H., Li, L., Chen, R. & Guo, S. Ionogel electrolytes for high‐performance lithium batteries: a review. Adv. Energy Mater. 8, 1702675 (2018).
Chen, N. et al. Biomimetic ant-nest ionogel electrolyte boosts the performance of dendrite-free lithium batteries. Energy Environ. Sci. 10, 1660–1667 (2017).
Hyun, W. J., Thomas, C. M. & Hersam, M. C. Nanocomposite ionogel electrolytes for solid‐state rechargeable batteries. Adv. Energy Mater. 10, 2002135 (2020).
Shi, L. et al. Dielectric gels with ultra-high dielectric constant, low elastic modulus, and excellent transparency. NPG Asia Mater. 10, 821–826 (2018).
Liu, Y., He, K., Chen, G., Leow, W. R. & Chen, X. Nature-inspired structural materials for flexible electronic devices. Chem. Rev. 117, 12893–12941 (2017).
Shi, L. et al. Highly stretchable and transparent ionic conducting elastomers. Nat. Commun. 9, 2630 (2018).
Lei, Z. & Wu, P. A highly transparent and ultra-stretchable conductor with stable conductivity during large deformation. Nat. Commun. 10, 3429 (2019).
Cao, Z., Liu, H. & Jiang, L. Transparent, mechanically robust, and ultrastable ionogels enabled by hydrogen bonding between elastomers and ionic liquids. Mater. Horiz. 7, 912–918 (2020).
Zhang, L. M. et al. Self‐healing, adhesive, and highly stretchable ionogel as a strain sensor for extremely large deformation. Small 15, 1804651 (2019).
Li, T., Wang, Y., Li, S., Liu, X. & Sun, J. Mechanically robust, elastic, and healable ionogels for highly sensitive ultra‐durable ionic skins. Adv. Mater. 32, 2002706 (2020).
Correia, D. M. et al. Ionic liquid–polymer composites: a new platform for multifunctional applications. Adv. Funct. Mater. 30, 1909736 (2020).
Le Bideau, J., Viau, L. & Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 40, 907–925 (2011).
Yu, L. et al. Highly tough, Li‐metal compatible organic–inorganic double‐network solvate ionogel. Adv. Energy Mater. 9, 1900257 (2019).
Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter 10, 672–687 (2014).
Gong, J. P., Katsuyama, Y., Kurokawa, T. & Osada, Y. Double‐network hydrogels with extremely high mechanical strength. Adv. Mater. 15, 1155–1158 (2003).
Sun, J.-Y. et al. Highly stretchable and tough hydrogels. Nature 489, 133–136 (2012).
Zhang, H. J. et al. Tough physical double‐network hydrogels based on amphiphilic triblock copolymers. Adv. Mater. 28, 4884–4890 (2016).
Sato, K. et al. Phase‐separation‐induced anomalous stiffening, toughening, and self‐healing of polyacrylamide gels. Adv. Mater. 27, 6990–6998 (2015).
Gong, J. P. Why are double network hydrogels so tough? Soft Matter 6, 2583–2590 (2010).
Corkhill, P., Trevett, A. & Tighe, B. The potential of hydrogels as synthetic articular cartilage. Proc. Inst. Mech. Eng. H 204, 147–155 (1990).
Zhang, H. et al. Polyelectrolyte microcapsules as ionic liquid reservoirs within ionomer membrane to confer high anhydrous proton conductivity. J. Power Sources 279, 667–677 (2015).
Ueki, T. & Watanabe, M. Polymers in ionic liquids: dawn of neoteric solvents and innovative materials. Bull. Chem. Soc. Jpn 85, 33–50 (2012).
Ueki, T., Watanabe, M. & Lodge, T. P. Doubly thermosensitive self-assembly of diblock copolymers in ionic liquids. Macromolecules 42, 1315–1320 (2009).
Weng, D. et al. Polymeric complex-based transparent and healable ionogels with high mechanical strength and ionic conductivity as reliable strain sensors. ACS Appl. Mater. Interfaces 12, 57477–57485 (2020).
Sun, T. L. et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 12, 932–937 (2013).
Hu, X., Vatankhah‐Varnoosfaderani, M., Zhou, J., Li, Q. & Sheiko, S. S. Weak hydrogen bonding enables hard, strong, tough, and elastic hydrogels. Adv. Mater. 27, 6899–6905 (2015).
Kong, W. et al. Muscle‐inspired highly anisotropic, strong, ion‐conductive hydrogels. Adv. Mater. 30, 1801934 (2018).
Mredha, M. T. I. et al. A facile method to fabricate anisotropic hydrogels with perfectly aligned hierarchical fibrous structures. Adv. Mater. 30, 1704937 (2018).
Asaletha, R., Kumaran, M. & Thomas, S. Thermoplastic elastomers from blends of polystyrene and natural rubber: morphology and mechanical properties. Eur. Polym. J. 35, 253–271 (1999).
Bai, R., Yang, J. & Suo, Z. Fatigue of hydrogels. Eur. J. Mech. A 74, 337–370 (2019).
Acknowledgements
M.D. acknowledges support from the Coastal Studies Institute. J.H. acknowledges the support of the National Natural Science Foundation of China (11702207). We thank Prof. L. Cai for helpful discussion. We thank Mr M. Yang and Mr X. Chen for help with 3D printing. Nano-IR analysis was performed by W.Q. at the NanoEngineering Research Core Facility (NERCF), which is partially funded by the Nebraska Research Initiative.
Author information
Authors and Affiliations
Contributions
M.W., J.H. and M.D.D. conceived the idea. J.H. and M.D.D. supervised the project. M.W. carried out most of the experiments. P.Z. participated in the fracture energy measurements. M.S. and V.K.T. participated in the SEM measurements. M.S. participated in the SAXS measurements. J.L.T. contributed to the SAXS data processing and analysis. W.Q. conducted the nano-IR measurements. J.M. contributed to the demonstration of the falling metal ball. M.W., J.H., and M.D.D. wrote the paper, and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Xuanhe Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–17, Notes 1–4, Tables 1 and 2, Videos 1–5 and refs. 1–7.
Supplementary Video 1
This movie shows that the P(AAm0.8125-co-AA0.1875) ionogel is strong enough to lift a 1 kg weight, while the pure PAA and PAAm ionogel and P(AAm0.8125-co-AA0.1875) hydrogel break. Cm = 6 M, CMBAA = 0.1 mol%.
Supplementary Video 2
This movie demonstrates the ultra-tough properties of the gel when a metal ball drops on a membrane of P(AAm0.8125-co-AA0.1875) ionogel stretched across a rigid frame. The membrane (thickness = 0.5 mm) was glued to two polyacrylate clamps with a circular opening (diameter = 7 cm). A stainless steel ball with a diameter of 2.54 cm and mass of 64 g was dropped from a height of 2 m. Upon hitting the membrane, the ball bounced back and the membrane remained intact with small deformation, while the P(AAm0.8125-co-AA0.1875) hydrogel was stretched to rupture after large deformation. Cm = 6 M, CMBAA = 0.1 mol%.
Supplementary Video 3
This movie shows the excellent self-healing property of the P(AAm0.8125-co-AA0.1875) ionogel. The dog-bone samples were cut into half pieces and then the pieces from two different samples were put together to heal. After storing the sample at 80 °C for 1 h, the self-healed sample could lift the 1 kg weight. The copolymer ionogel samples were stained with methylene blue and rhodamine B. Cm = 6 M, CMBAA = 0.1 mol%.
Supplementary Video 4
This movie shows the excellent shape-memory properties of P(AAm0.8125-co-AA0.1875) ionogel by demonstrating the fast programming and recovery process. The ionogel sample was stained with rhodamine B for visualization. Cm = 6 M, CMBAA = 0.1 mol%.
Supplementary Video 5
This movie exhibits superb shape-memory behaviour of P(AAm0.8125-co-AA0.1875) ionogel using a more complicated six-layer structure of a ‘blooming flower’. Six layers of the copolymer ionogel samples were glued together to mimic the flower bud blooming process and the ionogels could fully recover within 25 s. The ionogel samples were stained with methylene blue. Cm = 6 M, CMBAA = 0.1 mol%.
Source data
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Rights and permissions
About this article
Cite this article
Wang, M., Zhang, P., Shamsi, M. et al. Tough and stretchable ionogels by in situ phase separation. Nat. Mater. 21, 359–365 (2022). https://doi.org/10.1038/s41563-022-01195-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-022-01195-4
This article is cited by
-
Connective tissue inspired elastomer-based hydrogel for artificial skin via radiation-indued penetrating polymerization
Nature Communications (2024)
-
Tear-resistant stretchy gels
Nature Materials (2024)
-
Self-compliant ionic skin by leveraging hierarchical hydrogen bond association
Nature Communications (2024)
-
A solid-state lithium-ion battery with micron-sized silicon anode operating free from external pressure
Nature Communications (2024)
-
Compliant Iontronic Triboelectric Gels with Phase-Locked Structure Enabled by Competitive Hydrogen Bonding
Nano-Micro Letters (2024)