Abstract
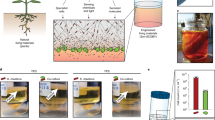
Engineered living materials could have the capacity to self-repair and self-replicate, sense local and distant disturbances in their environment, and respond with functionalities for reporting, actuation or remediation. However, few engineered living materials are capable of both responsivity and use in macroscopic structures. Here we describe the development, characterization and engineering of a fungal–bacterial biocomposite grown on lignocellulosic feedstocks that can form mouldable, foldable and regenerative living structures. We have developed strategies to make human-scale biocomposite structures using mould-based and origami-inspired growth and assembly paradigms. Microbiome profiling of the biocomposite over multiple generations enabled the identification of a dominant bacterial component, Pantoea agglomerans, which was further isolated and developed into a new chassis. We introduced engineered P. agglomerans into native feedstocks to yield living blocks with new biosynthetic and sensing–reporting capabilities. Bioprospecting the native microbiota to develop engineerable chassis constitutes an important strategy to facilitate the development of living biomaterials with new properties and functionalities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The sequencing data generated for this study are deposited in the NCBI SRA, accession number PRJNA673748. Metagenomic samples were classified against the SILVA database v.132 and RDP Classifier training set 17 (16S) and the UNITE database vl.12.17 (ITS). Source data are provided with this paper.
Code availability
The code used in the development of the signal propagation system design described in Fig. 4 is available at https://github.com/wanglabcumc/FungalBiocomposites.
References
Haneef, M. et al. Advanced materials from fungal mycelium: fabrication and tuning of physical properties. Sci. Rep. 7, 41292 (2017).
Jones, M., Mautner, A., Luenco, S., Bismarck, A. & John, S. Engineered mycelium composite construction materials from fungal biorefineries: a critical review. Mater. Des. 187, 108397 (2020).
Elsacker, E. et al. A comprehensive framework for the production of mycelium-based lignocellulosic composites. Sci. Total Environ. 725, 138431 (2020).
Silverman, J., Cao, H. T. & Cobb, K. Development of mushroom mycelium composites for footwear products. Cloth. Text. Res. J. 38, 119–133 (2020).
Attias, N. et al. Mycelium bio-composites in industrial design and architecture: comparative review and experimental analysis. J. Clean. Prod. 246, 119037 (2020).
Gilbert, C. & Ellis, T. Biological engineered living materials: growing functional materials with genetically programmable properties. ACS Synth. Biol. 8, 1–15 (2019).
Nguyen, P. Q., Courchesne, N. M. D., Duraj-Thatte, A., Praveschotinunt, P. & Joshi, N. S. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv. Mater. 30, e1704847 (2018).
Townsend-Nicholson, A. & Jayasinghe, S. N. Cell electrospinning: a unique biotechnique for encapsulating living organisms for generating active biological microthreads/scaffolds. Biomacromolecules 7, 3364–3369 (2006).
Gonzalez, L. M., Mukhitov, N. & Voigt, C. A. Resilient living materials built by printing bacterial spores. Nat. Chem. Biol. 16, 126–133 (2020).
Charrier, M. et al. Engineering the S-layer of Caulobacter crescentus as a foundation for stable, high-density, 2D living materials. ACS Synth. Biol. 8, 181–190 (2019).
Nguyen, P. Q., Botyanszki, Z., Tay, P. K. R. & Joshi, N. S. Programmable biofilm-based materials from engineered curli nanofibres. Nat. Commun. 5, 4945 (2014).
Chen, A. Y. et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat. Mater. 13, 515–523 (2014).
Walker, K. T., Goosens, V. J., Das, A., Graham, A. E. & Ellis, T. Engineered cell-to-cell signalling within growing bacterial cellulose pellicles. Microb. Biotechnol. 12, 611–619 (2019).
Caro-Astorga, J., Walker, K. T., Herrera, N., Lee, K.-Y. & Ellis, T. Bacterial cellulose spheroids as building blocks for 3D and patterned living materials and for regeneration. Nat. Commun. 12, 5027 (2021).
Gerber, L. C., Koehler, F. M., Grass, R. N. & Stark, W. J. Incorporation of penicillin-producing fungi into living materials to provide chemically active and antibiotic-releasing surfaces. Angew. Chem. Int. Ed. 51, 11293–11296 (2012).
Seker, U. O., Chen, A. Y., Citorik, R. J. & Lu, T. K. Synthetic biogenesis of bacterial amyloid nanomaterials with tunable inorganic–organic interfaces and electrical conductivity. ACS Synth. Biol. 6, 266–275 (2017).
Tay, P. K. R., Nguyen, P. Q. & Joshi, N. S. A synthetic circuit for mercury bioremediation using self assembling functional amyloids. ACS Synth. Biol. 6, 1841–1850 (2017).
Liu, X. et al. Stretchable living materials and devices with hydrogel–elastomer hybrids hosting programmed cells. Proc. Natl Acad. Sci. USA 114, 2200–2205 (2017).
Chen, X., Mahadevan, L., Driks, A. & Sahin, O. Bacillus spores as building blocks for stimuli-responsive materials and nanogenerators. Nat. Nanotechnol. 9, 137–141 (2014).
Cheng, H. P., Wang, P. M., Chen, J. W. & Wu, W. T. Cultivation of Acetobacter xylinum for bacterial cellulose production in a modified airlift reactor. Biotechnol. Appl. Biochem. 35, 125–132 (2002).
Dorval Courchesne, N.-M., Duraj-Thatte, A., Tay, P. K. R., Nguyen, P. Q. & Joshi, N. S. Scalable production of genetically engineered nanofibrous macroscopic materials via filtration. ACS Biomater. Sci. Eng. 3, 733–741 (2016).
Heveran, C. M. et al. Biomineralization and successive regeneration of engineered living building materials. Matter 2, 481–494 (2020).
Castro-Alonso, M. J. et al. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front. Mater. 6, 126 (2019).
BioMASON https://biomason.com (2020).
Botusharova, S., Gardner, D. & Harbottle, M. Augmenting microbially induced carbonate precipitation of soil with the capability to self-heal. J. Geotech. Geoenviron. Eng. 146, 04020010 (2020).
Chen, Y., Peng, R. & You, Z. Origami of thick panels. Science 349, 396–400 (2015).
Pinto, P. A. et al. Influence of ligninolytic enzymes on straw saccharification during fungal pretreatment. Bioresour. Technol. 111, 261–267 (2012).
Walterson, A. M. & Stavrinides, J. Pantoea: insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiol. Rev. 39, 968–984 (2015).
Sheth, R. U., Cabral, V., Chen, S. P. & Wang, H. H. Manipulating bacterial communities by in situ microbiome engineering. Trends Genet. 32, 189–200 (2016).
McKenzie, G. J. & Craig, N. L. Fast, easy and efficient: site-specific insertion of transgenes into Enterobacterial chromosomes using Tn7 without need for selection of the insertion event. BMC Microbiol. 6, 39 (2006).
Furubayashi, M. et al. A highly selective biosynthetic pathway to non-natural C50 carotenoids assembled from moderately selective enzymes. Nat. Commun. 6, 7534 (2015).
Ji, B. W. et al. Quantifying spatiotemporal variability and noise in absolute microbiota abundances using replicate sampling. Nat. Methods 16, 731–736 (2019).
Dong, Y. H., Xu, J. L., Li, X. Z. & Zhang, L. H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl Acad. Sci. USA 97, 3526–3531 (2000).
Sheth, R. U., Yim, S. S., Wu, F. L. & Wang, H. H. Multiplex recording of cellular events over time on CRISPR biological tape. Science 358, 1457–1461 (2017).
Lee, J. W., Chan, C. T. Y., Slomovic, S. & Collins, J. J. Next-generation biocontainment systems for engineered organisms. Nat. Chem. Biol. 14, 530–537 (2018).
Martinez, L. M., Martinez, A. & Gosset, G. Production of melanins with recombinant microorganisms. Front. Bioeng. Biotechnol. 7, 285 (2019).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Callahan, B. Silva taxonomic training data formatted for DADA2. Silva v.132. Zenodo https://doi.org/10.5281/zenodo.1172783 (2018).
Nilsson, R. H. et al. The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264 (2019).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007).
McMurdie, P. J. & Holmes, S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 (2019).
Ronda, C., Chen, S. P., Cabral, V., Yaung, S. J. & Wang, H. H. Metagenomic engineering of the mammalian gut microbiome in situ. Nat. Methods 16, 167–170 (2019).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595 (2017).
Seemann, T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Yoon, S. H., Ha, S. M., Lim, J., Kwon, S. & Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Ant. Van Leeuw. 110, 1281–1286 (2017).
Blin, K. et al. The antiSMASH database version 2: a comprehensive resource on secondary metabolite biosynthetic gene clusters. Nucleic Acids Res. 47, D625–D630 (2019).
Zhang, H. et al. dbCAN2: a meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 46, W95–W101 (2018).
Alcock, B. P. et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 48, D517–D525 (2020).
Johns, N. I. et al. Metagenomic mining of regulatory elements enables programmable species-selective gene expression. Nat. Methods 15, 323–329 (2018).
Acknowledgements
We thank the members of Wang Lab for comments and discussion on this work and manuscript. This work is supported by DARPA (HR0011-17-C-0068, W911NF-17-2-0077) and NSF (CCF-1807575). H.H.W. acknowledges additional funding from NSF (MCB-2032259, MCB-2025515, MCB‐1453219), NIH (1R01AI132403, 1R01DK118044) and the Burroughs Wellcome Fund (PATH1016691). R.M.M. thanks personal support from X. Weng and financial support from the Dean’s Fellowship from the Graduate School of Arts and Sciences of Columbia University. M.R. is supported by an NSF Graduate Research Fellowship (DGE-1644869).
Author information
Authors and Affiliations
Contributions
R.M.M., M.L, C.V., D.S. and H.H.W. developed the initial concept. R.M.M., M.L., N.M., T.S., D.M., H.C., A.K., M. Richardson and M. Reitman performed experiments and analysed the results. M. Richardson, R.M.M. and C.M. analysed the metagenomic and whole genome sequencing data. The overall project was supervised by C.V., D.S. and H.H.W. The manuscript was drafted by R.M.M. and H.H.W. with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
M.L., M. Reitman and D.S. are employees of Ecovative Design, at the time of study. The authors declare no additional competing interests.
Additional information
Peer review information Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Growth characteristics of Ganoderma sp. in the living biocomposite.
(a) The level of moisture, pH, and ergosterol, which is a measure of fungal biomass, was quantified over the course of a full cycle of biomaterial generation and subsequent iterative replication in Generation 2 of reground biomaterial.
Extended Data Fig. 2 Representative strength characterization for fungal biocomposites.
(a) Representative three-point bend test results for living material blocks in two orientations. Note the anisotropy in the results, presumably due to differences in fungal growth due to aeration or other factors at the top of the block mold, even when lidded. (b) Representative compressive strength test results for living material blocks.
Extended Data Fig. 3 Foldable biocomposite designs and generation of pop-up structures.
(a) The design process for complex origami-inspired structures, beginning with conceptual models, followed by in silico modeling, simulation, layout, and fabrication to produce a final object. An arch design is first grown on a flat surface using flat-pack molds along with the appropriate connecting joints and folding edges on the top and bottom surfaces using flexible matting. (b) The arch is kinematically erected via a pop-up assembly protocol, and the joints are allowed to naturally fuse, thus stabilizing the arch structure into its final designed form. (c) Compressive strength of hemp-grown fungal biocomposite over four generations. Bar graphs shows the mean of and error bars show the standard deviation (SD). (d) Replated and regrown living material after a year of ambient desiccation. The Ganoderma sp. is still viable, and easily recultivated, especially with kanamycin antibiotics and Rose Bengal as a mold suppressor. Blocks grown using this material as an inoculum, however, are prone to contamination.
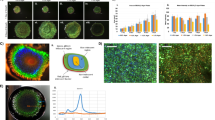
Extended Data Fig. 4 Microbiome characteristics of the living biocomposite.
Microbiome characteristics of the living biocomposite (a) Clustered heatmap of the bacterial abundance in biomaterial grown using different feedstocks and at different inoculation ratios (% sterile inoculum to % unsterile raw feedstock). Heatmap is in log10 scale in relative bacterial abundance. (b) Bacterial microbiome in a grown hemp block sampled at different spatial locations. At least 5 replicates per sampling location were analyzed (n = 5). (c) Analysis of 16 S reads at each spatially sampled location of a growing hemp block over 15-days, showing reproducible dominance of Pantoea spp.
Extended Data Fig. 5 Strain isolation from hemp feedstock.
(a) Unrooted tree of 33 isolates from hemp feedstock, mapped to the SILVA 16 S database. (b) Table of isolates and closest BLAST match against the NCBI 16S database. Isolates were either plated immediately from PBS-washed feedstock supernatant or allowed to wash and recover overnight before plating, which enriched for Pantoea spp. Note: Isolate ID #30 is annotated as Pantoea ananatis by NCBI 16S database, but was subsequently confirmed to be P. agglomerans and designated as P. agglomerans ELM1 throughout the study.
Extended Data Fig. 6 Growth characteristics of P. agglomerans ELM1.
(a) P. agglomerans ELM1 growth on standard LB rich media at 30 °C, as well as on a boiled hemp media derived from the same material feedstock from which the strain was isolated. Error bars show standard error of the mean (SEM) of three biological replicates (n = 3). (b) OD600 of P. agglomerans ELM1 in M9 minimal media on a number of carbon sources showing modest growth after 24 hours at 30 °C. Error bars show SEM. (c) Antibiotic susceptibility of P. agglomerans ELM1 (top) and their liquid growth profiles at different antibiotic concentrations (bottom). Error bars show SEM of three biological replicates (n = 3).
Extended Data Fig. 7 Complete genome of Pantoea agglomerans ELM1.
(a) The complete and closed genome of P. agglomerans ELM1 as well as two native plasmids. (b) Notable biosynthetic gene clusters present in the P. agglomerans ELM1 genome, including two pigment-producing biosynthetic clusters, which may be the source of the yellow tint of P. agglomerans ELM1 colonies, as well as an antibiotic-producing cluster and two quorum-sensing clusters that may contribute to P. agglomerans ELM1 capacity to dominate the hemp biomaterial during the growth phase.
Extended Data Fig. 8 Introduction of plasmids and genomic integration in P. agglomerans ELM1.
(a) Plasmids can be readily transformed by electroporation into P. agglomerans ELM1 with standard protocols used in other Gram-negative Proteobacteria. (b) Plasmids can be efficiently transferred from donor E. coli bacteria into P. agglomerans ELM1 via bacterial conjugation using the RK2 system in a dosage dependent manner. The conjugation efficiency as measured by the number of transconjugants found compared to total recipients is shown. All data are triplicate biological samples (n = 3) with the mean shown as a red line. (c) Schematic of the pGRG vector, target genomic site at attTn7, and resulting integrant in the genome. (d) The Tn7 integration locus (attTn7), present in many Enterobacteria, is also found in P. agglomerans ELM1. Sequence verification of three colonies chosen from an integration experiment in P. agglomerans ELM1 using pGRG36, validating successful integration into the attTn7 site.
Extended Data Fig. 9 Promoter characterization and canthaxanthin and violacein mass spectroscopy in P. agglomerans ELM1.
(a) A set of constitutive promoters was cloned upstream of a GFP fluorescent reporter and transformed into P. agglomerans ELM1. Promoter activity as measured by Relative Fluorescence Units normalized to OD600 (RFU/OD) after 17 hours of growth in P. agglomerans ELM1 using a platereader. (b) Cell extracts of P. agglomerans ELM1 with colors characteristic of Canthaxanthin (left) and violacein (right) production. (c) Mass spectrometry of putatively canthaxanthin producing P. agglomerans ELM1 showing a single, clean absorbance spike at ~16.7 minute elution time and the corresponding mass spectra. (d) Violacein producer mass spec with a single, clean absorbance peak at 6.5 minute elution time and the corresponding mass spectra.
Extended Data Fig. 10 Quorum sensing molecule-responsive living material blocks.
(a) Absolute abundance sequencing of bacterial species in a growing living material block. Excluding Pantoea species from the analysis reveals that P. agglomerans ELM1 spike-in appears to outcompete other bacterial species and suppresses non-Pantoea bacterial density. (b) Spiking in an engineered P. agglomerans ELM1 strain carrying a violacein-producing plasmid pNM175 results in purple blocks with no noticeable decrease in Ganoderma growth. (c) When a P. agglomerans strain expressing mCherry in response to an AHL signal is spiked into the living material, blocks become responsive to AHL dosed into the material when blocks are generated. (d) Inclusion of the receiver/propagator strain into living material blocks renders the material sensitive to AHL added to the surface of the block at block generation time, but the output fluorescence is fainter compared to constitutive mCherry expression.
Supplementary information
Supplementary Information
Supplementary Data Tables 1–8.
Supplementary Data 1
mCherry AHL-producer plasmid map.
Supplementary Data 2
AHL-feed-forward-responsive plasmid map.
Supplementary Data 3
AHL-producer plasmid map.
Supplementary Data 4
AHL-responsive plasmid map.
Supplementary Data 5
Raw data for Extended Data Fig. 9 and Fig. 3d.
Source data
Source Data Fig. 1
Raw data for Fig. 1b,c.
Source Data Fig. 2
Raw data for Fig. 2f.
Source Data Fig. 3
Raw data for Fig. 3b,c.
Source Data Extended Data Fig. 1
Raw data for Extended Data Fig. 1.
Source Data Extended Data Fig. 3
Raw data for Extended Data Fig. 3c.
Source Data Extended Data Fig. 6
Raw data for Extended Data Fig. 6a,b.
Source Data Extended Data Fig. 8
Raw data for Extended Data Fig. 8a,b.
Rights and permissions
About this article
Cite this article
McBee, R.M., Lucht, M., Mukhitov, N. et al. Engineering living and regenerative fungal–bacterial biocomposite structures. Nat. Mater. 21, 471–478 (2022). https://doi.org/10.1038/s41563-021-01123-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01123-y
This article is cited by
-
Accelerating the design of pili-enabled living materials using an integrative technological workflow
Nature Chemical Biology (2024)
-
Synthetic microbiology in sustainability applications
Nature Reviews Microbiology (2024)
-
Structural engineered living materials
Nano Research (2024)
-
3D bioprinting of microorganisms: principles and applications
Bioprocess and Biosystems Engineering (2024)
-
Engineered living materials for sustainable and resilient architecture
Nature Reviews Materials (2023)