Abstract
Implantable drug depots have the capacity to locally meet therapeutic requirements by maximizing local drug efficacy and minimizing potential systemic side effects. Tubular organs including the gastrointestinal tract, respiratory tract and vasculature all manifest with endoluminal disease. The anatomic distribution of localized drug delivery for these organs using existing therapeutic modalities is limited. Application of local depots in a circumferential and extended longitudinal fashion could transform our capacity to offer effective treatment across a range of conditions. Here we report the development and application of a kirigami-based stent platform to achieve this. The stents comprise a stretchable snake-skin-inspired kirigami shell integrated with a fluidically driven linear soft actuator. They have the capacity to deposit drug depots circumferentially and longitudinally in the tubular mucosa of the gastrointestinal tract across millimetre to multi-centimetre length scales, as well as in the vasculature and large airways. We characterize the mechanics of kirigami stents for injection, and their capacity to engage tissue in a controlled manner and deposit degradable microparticles loaded with therapeutics by evaluating these systems ex vivo and in vivo in swine. We anticipate such systems could be applied for a range of endoluminal diseases by simplifying dosing regimens while maximizing drug on-target effects through the sustained release of therapeutics and minimizing systemic side effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data supporting the results in this study are available within the paper and its Supplementary Information. Source data are provided with this paper.
Code availability
Abaqus scripts used for the numerical analyses are available from the corresponding author upon request.
References
Brem, H. et al. Biocompatibility of a biodegradable, controlled-release polymer in the rabbit brain. Sel. Cancer Ther. 5, 55–65 (1989).
Shatzel, J. et al. Drug eluting biliary stents to decrease stent failure rates: a review of the literature. World J. Gastrointest. Endosc. 8, 77–85 (2016).
Kong, Y. et al. Preparation and characterization of paclitaxel-loaded poly lactic acid-co-glycolic acid coating tracheal stent. Chin. Med. J. 127, 2236–2240 (2014).
Zhang, S. An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 7, 300ra128 (2015).
Kearney, C. J. & Mooney, D. J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 12, 1004–1017 (2013).
Stefanini, G. G. & Holmes, D. R. Drug therapy: drug-eluting coronary-artery stents. N. Engl. J. Med. 368, 254–265 (2013).
Martins, B. et al. Endoscopic management and prevention of migrated esophageal stents. World J. Gastrointest. Endosc. 6, 49–54 (2014).
Lee, P., Moore, R., Raizada, A. & Grotz, R. Small bowel perforation after duodenal stent migration: an interesting case of a rare complication. World J. Radiol. 3, 152–154 (2011).
Nelson, D. B. et al. Technology status evaluation report: injection needles. Gastrointest. Endosc. 50, 928–931 (1999).
Chung, S. S. et al. Randomised comparison between adrenaline injection alone and adrenaline injection plus heat probe treatment for actively bleeding ulcers. BMJ 314, 1307–1311 (1997).
Soehendra, N., Bohnaker, S. & Binmoeller, K. F. Nonvariceal upper gastrointestinal bleeding. New and alternative hemostatic techniques. Gastrointest. Endosc. Clin. North Am. 7, 641–657 (1997).
Pang, Y. et al. Endoscopically injectable shear-thinning hydrogels facilitating polyp removal. Adv. Sci. 6, 1901041 (2019) .
Usman, R. M., Jehangir, Q. & Bilal, M. Recurrent esophageal stricture secondary to pemphigus vulgaris: a rare diagnostic and therapeutic challenge. ACG Case Rep. J. 6, e00022 (2019).
Swaminath, A. & Lichtiger, S. Dilation of colonic strictures by intralesional injection of infliximab in patients with Crohn’s colitis. Inflamm. Bowel Dis. 14, 213–216 (2008).
Rafsanjani, A., Zhang, Y., Liu, B., Rubinstein, S. M. & Bertoldi, K. Kirigami skins make a simple soft actuator crawl. Sci. Robot. 3, eaar7555 (2018).
Tang, Y., Li, Y., Hong, Y., Yang, S. & Yin, J. Programmable active kirigami metasheets with more freedom of actuation. Proc. Natl Acad. Sci. USA 116, 26407–26413 (2019).
Fu, H. et al. Morphable 3D mesostructures and microelectronic devices by multistable buckling mechanics. Nat. Mater. 17, 268–276 (2018).
Morikawa, Y. et al. Donut-shaped stretchable kirigami: enabling electronics to integrate with the deformable muscle. Adv. Health. Mater. 8, 1900939 (2019).
Shyu, T. C. et al. A kirigami approach to engineering elasticity in nanocomposites through patterned defects. Nat. Mater. 14, 785–789 (2015).
Zheng, W. et al. Kirigami-inspired highly stretchable nanoscale devices using multidimensional deformation of monolayer MoS2. Chem. Mater. 30, 6063–6070 (2018).
Liu, Z. et al. Nano-kirigami with giant optical chirality. Sci. Adv. 4, eaat4436 (2018).
Wu, C., Wang, X., Lin, L., Guo, H. & Wang, Z. L. Paper-based triboelectric nanogenerators made of stretchable interlocking kirigami patterns. ACS Nano 10, 4652–4659 (2016).
Rafsanjani, A. & Bertoldi, K. Buckling-induced kirigami. Phys. Rev. Lett. 118, 084301 (2017).
Celli, P. et al. Shape-morphing architected sheets with non-periodic cut patterns. Soft Matter 14, 9744–9749 (2018).
Zhang, Y. et al. A mechanically driven form of kirigami as a route to 3D mesostructures in micro/nanomembranes. Proc. Natl Acad. Sci. USA 112, 11757–11764 (2015).
Babaee, S. et al. Bioinspired kirigami metasurfaces as assistive shoe grips. Nat. Biomed. Eng. 4, 778–786 (2020).
Connolly, F., Polygerinos, P., Walsh, C. J. & Bertoldi, K. Mechanical programming of soft actuators by varying fiber angle. Soft Robot. 2, 26–32 (2015).
Mintz, Y., Horgan, S., Cullen, J., Falor, E. & Talamini, M. A. Dual-lumen natural orifice translumenal endoscopic surgery (NOTES): a new method for performing a safe anastomosis. Surg. Endosc. 22, 348–351 (2008).
Tarnoff, M., Shikora, S. & Lembo, A. Acute technical feasibility of an endoscopic duodenal-jejunal bypass sleeve in a porcine model: a potentially novel treatment for obesity and type 2 diabetes. Surg. Endosc. 22, 772–776 (2008).
Leary, S. et al. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition (American Veterinary Medical Association, 2013).
Gómez-Gaete, C. et al. Development, characterization and in vitro evaluation of biodegradable rhein-loaded microparticles for treatment of osteoarthritis. Eur. J. Pharm. Sci. 96, 390–397 (2016).
Acknowledgements
We thank R. Langer for helpful discussion and support. We thank V. Spanoudaki and W. Huang for help with the micro-CT and histology images, A. Rafsanjani for productive discussions and all colleagues of the Langer and Traverso Laboratories for fruitful discussions. This work was funded in part by the Karl van Tassel (1925) Career Development Professorship and the Department of Mechanical Engineering, Massachusetts Institute of Technology.
Author information
Authors and Affiliations
Contributions
S.B., Y.S. and G.T. conceived and designed the research. S.B. and Y.S. performed the design, FE simulations, manufacturing, mechanical characterization and in vitro testing of the stent prototypes. S.T., J.E.C., K.I., A.M.H., M.A., S.B. and Y.S. performed the in vivo pig experiments. S.A. performed particle synthesis and characterization. K.H. and A.L. performed the biochemistry analysis. S.B., Y.S., S.A., M.W., M.A. and G.T. discussed and analysed the results and wrote the manuscript. All authors reviewed the manuscript and provided active and valuable feedback.
Corresponding author
Ethics declarations
Competing interests
S.B., Y.S., S.A., M.A. and G.T. are co-inventors on provisional patent application 63/041154 describing the technologies presented in this paper. Complete details of all relationships for profit and not for profit for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. The other authors declare no competing interests.
Additional information
Peer review Information Nature Materials thanks Edith Mathiowitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Methods and captions for Videos 1–9.
Supplementary Video 1
Deformation of the kirigami-based stent obtained using FE simulations.
Supplementary Video 2
Effect of the size of kirigami needles on deformation of the kirigami-based stents using FE simulations.
Supplementary Video 3
Effect of kirigami shell thickness on deformation of the kirigami-based stents using FE simulations.
Supplementary Video 4
Fluid-powered soft linear actuator.
Supplementary Video 5
Effect of kirigami surface thickness on deformation of the kirigami stents using experiments and FE simulations.
Supplementary Video 6
Effect of the size of kirigami needles on deformation of the kirigami stents using experiments and FE simulations.
Supplementary Video 7
Characterization of the fabricated oesophageal kirigami stent.
Supplementary Video 8
Spray coating of the kirigami stent.
Supplementary Video 9
Endoscopic evaluation of oesophagus after in vivo deployment of the kirigami stents in pigs.
Source data
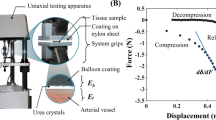
Source Data Fig. 1
Source data for numerical characterization of kirigami-based stents.
Source Data Fig. 2
Source data for experimental characterization of the oesophageal stent prototypes.
Source Data Fig. 3
Source data for evaluation of controlled penetration of the stent needles.
Source Data Fig. 4
Source data for extended drug release.
Rights and permissions
About this article
Cite this article
Babaee, S., Shi, Y., Abbasalizadeh, S. et al. Kirigami-inspired stents for sustained local delivery of therapeutics. Nat. Mater. 20, 1085–1092 (2021). https://doi.org/10.1038/s41563-021-01031-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01031-1
This article is cited by
-
Study of rhombic star-shaped honeycomb with tunable Poisson’s ratio and elastic modulus properties
Applied Physics A (2024)
-
Physics-aware differentiable design of magnetically actuated kirigami for shape morphing
Nature Communications (2023)
-
A reprogrammable mechanical metamaterial with origami functional-group transformation and ring reconfiguration
Nature Communications (2023)
-
A microexplosive shockwave-based drug delivery microsystem for treating hard-to-reach areas in the human body
Microsystems & Nanoengineering (2022)
-
Dynamic morphological transformations in soft architected materials via buckling instability encoded heterogeneous magnetization
Nature Communications (2022)