Abstract
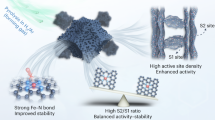
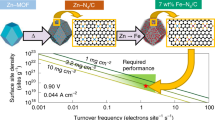
Replacing scarce and expensive platinum (Pt) with metal–nitrogen–carbon (M–N–C) catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells has largely been impeded by the low oxygen reduction reaction activity of M–N–C due to low active site density and site utilization. Herein, we overcome these limits by implementing chemical vapour deposition to synthesize Fe–N–C by flowing iron chloride vapour over a Zn–N–C substrate at 750 °C, leading to high-temperature trans-metalation of Zn–N4 sites into Fe–N4 sites. Characterization by multiple techniques shows that all Fe–N4 sites formed via this approach are gas-phase and electrochemically accessible. As a result, the Fe–N–C catalyst has an active site density of 1.92 × 1020 sites per gram with 100% site utilization. This catalyst delivers an unprecedented oxygen reduction reaction activity of 33 mA cm−2 at 0.90 V (iR-corrected; i, current; R, resistance) in a H2–O2 proton exchange membrane fuel cell at 1.0 bar and 80 °C.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this Article and its Supplementary Information. Additional data are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
References
Thompson, S. T. & Papageorgopoulos, D. Platinum group metal-free catalysts boost cost competitiveness of fuel cell vehicles. Nat. Catal. 2, 558–561 (2019).
Thompson, S. T. et al. Direct hydrogen fuel cell electric vehicle cost analysis: system and high-volume manufacturing description, validation, and outlook. J. Power Sources 399, 304–313 (2018).
Thompson, S. T. et al. ElectroCat: DOE’s approach to PGM-free catalyst and electrode R&D. Solid State Ion. 319, 68–76 (2018).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Li, J. et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells. Nat. Catal. 1, 935–945 (2018).
Zitolo, A. et al. Identification of catalytic sites in cobalt-nitrogen-carbon materials for the oxygen reduction reaction. Nat. Commun. 8, 957 (2017).
Zhang, H. et al. Single atomic iron catalysts for oxygen reduction in acidic media: particle size control and thermal activation. J. Am. Chem. Soc. 139, 14143–14149 (2017).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Proietti, E. et al. Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells. Nat. Commun. 2, 416 (2011).
Banham, D. et al. Critical advancements in achieving high power and stable nonprecious metal catalyst–based MEAs for real-world proton exchange membrane fuel cell applications. Sci. Adv. 4, eaar7180 (2018).
Serov, A. et al. Nano-structured non-platinum catalysts for automotive fuel cell application. Nano Energy 16, 293–300 (2015).
Wan, X. et al. Fe–N–C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells. Nat. Catal. 2, 259–268 (2019).
Li, J. et al. Structural and mechanistic basis for the high activity of Fe-N-C catalysts toward oxygen reduction. Energy Environ. Sci. 9, 2418–2432 (2016).
Zhang, H. et al. High-performance fuel cell cathodes exclusively containing atomically dispersed iron active sites. Energy Environ. Sci. 12, 2548–2558 (2019).
Gupta, S., Tryk, D., Bae, I., Aldred, W. & Yeager, E. Heat-treated polyacrylonitrile-based catalysts for oxygen electroreduction. J. Appl. Electrochem. 19, 19–27 (1989).
Li, J. et al. Evolution pathway from iron compounds to Fe1(II)–N4 sites through gas-phase iron during pyrolysis. J. Am. Chem. Soc. 142, 1417–1423 (2020).
Mineva, T. et al. Understanding active sites in pyrolyzed Fe–N–C catalysts for fuel cell cathodes by bridging density functional theory calculations and 57Fe Mössbauer spectroscopy. ACS Catal. 9, 9359–9371 (2019).
Zitolo, A. et al. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 14, 937–942 (2015).
Primbs, M. et al. Establishing reactivity descriptors for platinum group metal (PGM)-free Fe–N–C catalysts for PEM fuel cells. Energy Environ. Sci. 13, 2480–2500 (2020).
Sahraie, N. R. et al. Quantifying the density and utilization of active sites in non-precious metal oxygen electroreduction catalysts. Nat. Commun. 6, 8618 (2015).
Leonard, N. D. et al. Deconvolution of utilization, site density, and turnover frequency of Fe–nitrogen–carbon oxygen reduction reaction catalysts prepared with secondary N-precursors. ACS Catal. 8, 1640–1647 (2018).
Malko, D., Kucernak, A. & Lopes, T. In situ electrochemical quantification of active sites in Fe–N/C non-precious metal catalysts. Nat. Commun. 7, 13285 (2016).
Paulus, U. A. et al. Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 47, 3787–3798 (2002).
Gasteiger, H. A., Kocha, S. S., Sompalli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B Environ. 56, 9–35 (2005).
Luo, F. et al. P-block single-metal-site tin/nitrogen-doped carbon fuel cell cathode catalyst for oxygen reduction reaction. Nat. Mater. 19, 1215–1223 (2020).
Jia, Q. et al. Spectroscopic insights into the nature of active sites in iron–nitrogen–carbon electrocatalysts for oxygen reduction in acid. Nano Energy 29, 65–82 (2016).
Menga, D. et al. Active-site imprinting: preparation of Fe–N–C catalysts from zinc ion–templated ionothermal nitrogen-doped carbons. Adv. Energy Mater. 9, 1902412 (2019).
Li, J. et al. Volcano trend in electrocatalytic CO2 reduction activity over atomically dispersed metal sites on nitrogen-doped carbon. ACS Catal. 9, 10426–10439 (2019).
Wang, Q. et al. Evolution of Zn(II) single atom catalyst sites during the pyrolysis-induced transformation of ZIF-8 to N-doped carbons. Sci. Bull. 65, 1743–1751 (2020).
Artyushkova, K. Misconceptions in interpretation of nitrogen chemistry from X-ray photoelectron spectra. J. Vac. Sci. Technol. A 38, 031002 (2020).
Rustad, D. S. & Gregory, N. W. Vapor pressure of iron(III) chloride. J. Chem. Eng. Data 28, 151–155 (1983).
Shao, Y., Dodelet, J.-P., Wu, G. & Zelenay, P. PGM-free cathode catalysts for PEM fuel cells: a mini-review on stability challenges. Adv. Mater. 31, 1807615 (2019).
Osmieri, L., Cullen, D. A., Chung, H. T., Ahluwalia, R. K. & Neyerlin, K. C. Durability evaluation of a Fe–N–C catalyst in polymer electrolyte fuel cell environment via accelerated stress tests. Nano Energy 78, 105209 (2020).
Osmieri, L. et al. Status and challenges for the application of platinum group metal-free catalysts in proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 25, 100627 (2021).
Kramm, U. I., Ni, L. & Wagner, S. 57Fe Mössbauer spectroscopy characterization of electrocatalysts. Adv. Mater. 31, 1805623 (2019).
Kramm, U. I. et al. Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cells. Phys. Chem. Chem. Phys. 14, 11673–11688 (2012).
Li, J. et al. Identification of durable and non-durable FeNx sites in Fe–N–C materials for proton exchange membrane fuel cells. Nat. Catal. 4, 10–19 (2021).
Zelenay, P. ElectroCat (Electrocatalysis Consortium) Report No. LA-UR-20-24045 (Los Alamos National Laboratory, 2020).
Ferrandon, M. et al. Multitechnique characterization of a polyaniline–iron–carbon oxygen reduction catalyst. J. Phys. Chem. C 116, 16001–16013 (2012).
Dészi, I., Ouseph, P. J. & Thomas, P. M. Mössbauer study of FeCl2·6H2O and its decomposition product. Chem. Phys. Lett. 9, 390–392 (1971).
DeBenedetti, S., Lang, G. & Ingalls, R. Electric quadrupole splitting and the nuclear volume effect in the ions of Fe57. Phys. Rev. Lett. 6, 60–62 (1961).
Bach, R. D., Shobe, D. S., Schlegel, H. B. & Nagel, C. J. Thermochemistry of iron chlorides and their positive and negative ions. J. Phys. Chem. 100, 8770–8776 (1996).
Kim, D. H. et al. Selective electrochemical reduction of nitric oxide to hydroxylamine by atomically dispersed iron catalyst. Nat. Commun. 12, 1856 (2021).
Luo, F. et al. Accurate evaluation of active-site density (SD) and turnover frequency (TOF) of PGM-free metal–nitrogen-doped carbon (MNC) electrocatalysts using CO cryo adsorption. ACS Catal. 9, 4841–4852 (2019).
Mehmood, A. et al. Facile metal coordination of active site imprinted nitrogen doped carbons for the conservative preparation of non-noble metal oxygen reduction electrocatalysts. Adv. Energy Mater. 8, 1701771 (2018).
Hwang, D.-M. D. in Graphite Intercalation Compounds I: Structure and Dynamics (eds Zabel, H. & Solin, S.) 247–281 (Springer, 1990).
Zhang, B., Song, J., Yang, G. & Han, B. Large-scale production of high-quality graphene using glucose and ferric chloride. Chem. Sci. 5, 4656–4660 (2014).
Lutfullin, M. A., Shornikova, O. N., Sorokina, N. E. & Avdeev, V. V. Interaction of FeCl3-intercalated graphite with intercalants of different strengths. Inorg. Mater. 50, 29–34 (2014).
Jia, Q. et al. Activity descriptor identification for oxygen reduction on platinum-based bimetallic nanoparticles: in situ observation of the linear composition–strain–activity relationship. ACS Nano 9, 387–400 (2015).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Acknowledgements
This work was supported by the DOE under award number DE-EE0008416 (Q.J.) and DE-EE0008075 (H.X.). We acknowledge the support from the DOE, Energy Efficiency and Renewable Energy, Hydrogen and Fuel Cell Technologies Office through the Electrocatalysis Consortium (ElectroCat) and the DOE programme and technology managers, D. Papageorgopoulos, D. Peterson and N. Garland. The ex situ XAS experiments at the Zn K edge were performed at the Advanced Photon Source, a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. The operation of the Materials Research Collaborative Access Team at the Advanced Photon Source is supported by the DOE and the Materials Research Collaborative Access Team member institutions. The rest of the XAS data were collected at beamlines 6-BM, 7-BM and 8-ID (ISS) of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. AC-STEM was conducted at the Center for Nanophase Materials Sciences located at Oak Ridge National Laboratory, which is a DOE Office of Science User Facility. The submitted manuscript was created, in part, by UChicago Argonne, Operator of Argonne National Laboratory (‘Argonne’), a DOE Office of Science laboratory, operated under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
Q.J., D.J.M. and F.J. conceived the project. Q.J. and J.L. conceived and designed the CVD method. Q.J., J.L., D.J.M., F.J. and L.J. developed the CVD method. L.J. synthesized the FeNC-CVD-T catalysts; Q.J. and S.M. supervised and advised the synthesis. L.J. conducted the RDE, Brunauer–Emmett–Teller, X-ray diffraction, TEM, SEM and inductively coupled plasma atomic emission spectroscopy. L.J., Q.S., L.L.R., T.S., E.L. and Q.J. conducted the XAS on the FeNC-CVD-750. T.S. and D.J.M. conducted the XAS on the Zn–N–C and ZIF-8 at the Zn K edge. Q.J. analysed the XAS data. M.T.S., J.L. and F.J. conducted the Mössbauer and the fitting. Z.Z. and Y.H. conducted the XPS and fitting. F.Y., S.Z. and H.X. conducted the PEMFC operation and data analysis. D.A.C. conducted the ADF-STEM, electron energy-loss spectroscopy and data analysis. M.F. and D.J.M. conducted the temperature-programmed reaction studies. J.H.P., M.F. and D.J.M. conducted the NO stripping and data analysis. L.J. did the nitrite stripping. Q.J., F.J., D.J.M. and L.J. wrote the manuscript and prepared the figures.
Corresponding authors
Ethics declarations
Competing interests
L.J., S.M. and Q.J. have filed a full patent application (no. PCT/US2020/058362) based on the results of this manuscript. The inventors are Q.J., L.J., and S.M. The application is currently pending. The CVD method for the synthesis of M–N–C catalysts is covered by this patent application. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–6, Figs. 1–15, Tables 1–6 and references.
Source data
Source Data Fig. 1
Source data used for plotting the curves and symbols.
Source Data Fig. 2
Source data used for plotting the curves and symbols.
Source Data Fig. 3
Source data used for plotting the curves and symbols.
Source Data Fig. 4
Source data used for plotting the curves and symbols.
Rights and permissions
About this article
Cite this article
Jiao, L., Li, J., Richard, L.L. et al. Chemical vapour deposition of Fe–N–C oxygen reduction catalysts with full utilization of dense Fe–N4 sites. Nat. Mater. 20, 1385–1391 (2021). https://doi.org/10.1038/s41563-021-01030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01030-2
This article is cited by
-
Tailoring coordination environments of single-atom electrocatalysts for hydrogen evolution by topological heteroatom transfer
Nature Communications (2024)
-
Simultaneous integration of Fe clusters and NiFe dual single atoms in nitrogen-doped carbon for oxygen reduction reaction
Nano Research (2024)
-
A general approach to 3D-printed single-atom catalysts
Nature Synthesis (2023)
-
High-entropy single-atom activated carbon catalysts for sustainable oxygen electrocatalysis
Nature Sustainability (2023)
-
Preparation of highly dispersed FeNx active sites for oxygen reduction reaction electrocatalyst by electrospinning and complexation
Ionics (2023)