Abstract
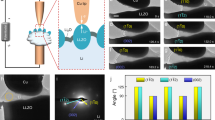
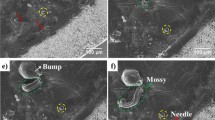
Lithium dendrite (filament) propagation through ceramic electrolytes, leading to short circuits at high rates of charge, is one of the greatest barriers to realizing high-energy-density all-solid-state lithium-anode batteries. Utilizing in situ X-ray computed tomography coupled with spatially mapped X-ray diffraction, the propagation of cracks and the propagation of lithium dendrites through the solid electrolyte have been tracked in a Li/Li6PS5Cl/Li cell as a function of the charge passed. On plating, cracking initiates with spallation, conical ‘pothole’-like cracks that form in the ceramic electrolyte near the surface with the plated electrode. The spallations form predominantly at the lithium electrode edges where local fields are high. Transverse cracks then propagate from the spallations across the electrolyte from the plated to the stripped electrode. Lithium ingress drives the propagation of the spallation and transverse cracks by widening the crack from the rear; that is, the crack front propagates ahead of the Li. As a result, cracks traverse the entire electrolyte before the Li arrives at the other electrode, and therefore before a short circuit occurs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Supporting research data have been deposited in the Oxford Research Archive and are available at https://doi.org/10.5287/bodleian:9Rn6n6o15.
References
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Pellegrini, V., Bodoardo, S., Brandell, D. & Edström, K. Challenges and perspectives for new material solutions in batteries. Solid State Commun. 303–304, 113733 (2019).
Palacín, M. R. Recent advances in rechargeable battery materials: a chemist’s perspective. Chem. Soc. Rev. 38, 2565–2575 (2009).
Famprikis, T., Canepa, P., Dawson, J. A., Islam, M. S. & Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18, 1278–1291 (2019).
Fincher, C. D., Ojeda, D., Zhang, Y., Pharr, G. M. & Pharr, M. Mechanical properties of metallic lithium: from nano to bulk scales. Acta Mater. 186, 215–222 (2020).
Chen, Y. et al. Li metal deposition and stripping in a solid-state battery via Coble creep. Nature 578, 251–255 (2020).
van den Broek, J., Afyon, S. & Rupp, J. L. M. Interface-engineered all-solid-state Li-ion batteries based on garnet-type fast Li+ conductors. Adv. Energy Mater. 6, 1600736 (2016).
Yang, C. et al. Continuous plating/stripping behavior of solid-state lithium metal anode in a 3D ion-conductive framework. Proc. Natl Acad. Sci. USA 115, 3770–3775 (2018).
Masias, A., Felten, N., Garcia-Mendez, R., Wolfenstine, J. & Sakamoto, J. Elastic, plastic, and creep mechanical properties of lithium metal. J. Mater. Sci. 54, 2585–2600 (2019).
Han, F., Yue, J., Zhu, X. & Wang, C. Suppressing Li dendrite formation in Li2S-P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 8, 1703644 (2018).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Lotsch, B. V. & Maier, J. Relevance of solid electrolytes for lithium-based batteries: a realistic view. J. Electroceram. 38, 128–141 (2017).
Pang, Q., Liang, X., Shyamsunder, A. & Nazar, L. F. An in vivo formed solid electrolyte surface layer enables stable plating of Li metal. Joule 1, 871–886 (2017).
Kerman, K., Luntz, A., Viswanathan, V., Chiang, Y.-M. & Chen, Z. Review—practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 164, A1731–A1744 (2017).
LePage, W. S. et al. Lithium mechanics: roles of strain rate and temperature and implications for lithium metal batteries. J. Electrochem. Soc. 166, A89–A97 (2019).
Monroe, C. & Newman, J. The effect of interfacial deformation on electrodeposition kinetics. J. Electrochem. Soc. 151, A880–A886 (2004).
Monroe, C. & Newman, J. The impact of elastic deformation on deposition kinetics at lithium/polymer interfaces. J. Electrochem. Soc. 152, A396–A404 (2005).
Sharafi, A., Meyer, H. M., Nanda, J., Wolfenstine, J. & Sakamoto, J. Characterizing the Li–Li7La3Zr2O12 interface stability and kinetics as a function of temperature and current density. J. Power Sources 302, 135–139 (2016).
Marbella, L. E. et al. 7Li NMR chemical shift imaging to detect microstructural growth of lithium in all-solid-state batteries. Chem. Mater. 31, 2762–2769 (2019).
Wu, B. et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 11, 1803–1810 (2018).
Swamy, T. et al. Lithium metal penetration induced by electrodeposition through solid electrolytes: example in single-crystal Li6La3ZrTaO12 garnet. J. Electrochem. Soc. 165, A3648–A3655 (2018).
Kazyak, E. et al. Li penetration in ceramic solid electrolytes: operando microscopy analysis of morphology, propagation, and reversibility. Matter 2, 1025–1048 (2020).
Manalastas, W. et al. Mechanical failure of garnet electrolytes during Li electrodeposition observed by in-operando microscopy. J. Power Sources 412, 287–293 (2019).
Tippens, J. et al. Visualizing chemomechanical degradation of a solid-state battery electrolyte. ACS Energy Lett. 4, 1475–1483 (2019).
Seitzman, N. et al. Toward all-solid-state lithium batteries: three-dimensional visualization of lithium migration in β-Li3PS4 ceramic electrolyte. J. Electrochem. Soc. 165, A3732–A3737 (2018).
Doux, J. et al. Stack pressure considerations for room temperature all-solid-state lithium metal batteries. Adv. Energy Mater. 10, 1903253 (2019).
Spencer Jolly, D. et al. Sodium/Na β″ alumina interface: effect of pressure on voids. ACS Appl. Mater. Interfaces 12, 678–685 (2020).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Boulineau, S., Courty, M., Tarascon, J. M. & Viallet, V. Mechanochemical synthesis of Li-argyrodite Li 6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application. Solid State Ion. 221, 1–5 (2012).
Wenzel, S., Sedlmaier, S. J., Dietrich, C., Zeier, W. G. & Janek, J. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018).
Yu, C., van Eijck, L., Ganapathy, S. & Wagemaker, M. Synthesis, structure and electrochemical performance of the argyrodite Li6PS5Cl solid electrolyte for Li-ion solid state batteries. Electrochim. Acta 215, 93–99 (2016).
Lee, H. et al. Advances and prospects of sulfide all-solid-state lithium batteries via one-to-one comparison with conventional liquid lithium ion batteries. Adv. Mater. 31, 1900376 (2019).
Krauskopf, T. et al. Lithium-metal growth kinetics on LLZO garnet-type solid electrolytes. Joule 3, 2030–2049 (2019).
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Wenzel, S. et al. Interfacial reactivity benchmarking of the sodium ion conductors Na3PS4 and sodium β-alumina for protected sodium metal anodes and sodium all-solid-state batteries. ACS Appl. Mater. Interfaces 8, 28216–28224 (2016).
Zhu, Y., He, X. & Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Bay, B. K., Smith, T. S., Fyhrie, D. P. & Saad, M. Digital volume correlation: three-dimensional strain mapping using X-ray tomography. Exp. Mech. 39, 217–226 (1999).
Eastwood, D. S. et al. Lithiation-induced dilation mapping in a lithium-ion battery electrode by 3D X-ray microscopy and digital volume correlation. Adv. Energy Mater. 4, 1300506 (2014).
Pietsch, P. & Wood, V. X-ray tomography for lithium ion battery research: a practical guide. Annu. Rev. Mater. Res. 47, 451–479 (2017).
Maire, E., Merle, P., Peix, G., Baruchel, J. & Buffière, J. Y. X-Ray Tomography in Material Science (Hermes Science Publications, 2000).
Athanasiou, C. E., Jin, M. Y., Ramirez, C., Padture, N. P. & Sheldon, B. W. High-toughness inorganic solid electrolytes via the use of reduced graphene oxide. Matter 3, 212–229 (2020).
Klinsmann, M., Hildebrand, F. E., Ganser, M. & McMeeking, R. M. Dendritic cracking in solid electrolytes driven by lithium insertion. J. Power Sources 442, 227226 (2019).
Bucci, G. & Christensen, J. Modeling of lithium electrodeposition at the lithium/ceramic electrolyte interface: the role of interfacial resistance and surface defects. J. Power Sources 441, 227186 (2019).
Barroso-Luque, L., Tu, Q. & Ceder, G. An analysis of solid-state electrodeposition-induced metal plastic flow and predictions of stress states in solid ionic conductor defects. J. Electrochem. Soc. 167, 020534 (2020).
Tang, M., Albertus, P. & Newman, J. Two-dimensional modeling of lithium deposition during cell charging. J. Electrochem. Soc. 156, A390–A399 (2009).
Ren, Y., Shen, Y., Lin, Y. & Nan, C. W. Microstructure manipulation for enhancing the resistance of garnet-type solid electrolytes to ‘short circuit’ by Li metal anodes. ACS Appl. Mater. Interfaces 11, 5928–5937 (2019).
Shen, F., Dixit, M. B., Xiao, X. & Hatzell, K. B. Effect of pore connectivity on Li dendrite propagation within LLZO electrolytes observed with synchrotron X-ray tomography. ACS Energy Lett. 3, 1056–1061 (2018).
Paganin, D., Mayo, S. C., Gureyev, T. E., Miller, P. R. & Wilkins, S. W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 206, 33–40 (2002).
Marone, F. & Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Radiat. 19, 1029–1037 (2012).
Acknowledgements
P.G.B. is indebted to the Faraday Institution All-Solid-State Batteries with Li and Na Anodes (FIRG007, FIRG008), as well as the Engineering and Physical Sciences Research Council, Enabling Next Generation Lithium Batteries (EP/M009521/1), the University of Oxford experimental equipment upgrade (EP/M02833X/1) and the Henry Royce Institute for Advanced Materials (EP/R0066X/1, EP/S019367/1, EP/R010145/1) for financial support. G.L. and C.W.M. acknowledge the Faraday Institution Multiscale Modelling (FIRG003) and the UK Industrial Strategy Challenge Fund: Materials Research Hub for Energy Conversion, Capture, and Storage, under grant EP/R023581/1, for financial support. J.I. is supported by the Swiss National Science Foundation (no. PZ00P2_179886). We thank Paul Scherrer Institut, Villigen, Switerland, and Diamond Light Source, Harwell, United Kingdom, for provision of synchrotron radiation beam time (experiment no. 20182142) at the TOMCAT beamline X02DA of the Swiss Light Source, and beam time (experiment no. EE20795-1) at the I12 beamline of the Diamond Light Source. We acknowledge technical and experimental support at the TOMCAT by A. Bonnin and J. Ihli, and at the I12 by O. Magdysyuk.
Author information
Authors and Affiliations
Contributions
Z.N. contributed to all aspects of the research. Z.N., D.S.J., J.I. and A.B. carried out the in situ phase-contrast synchrotron XCT. Z.N., D.S.J., R.D.M., S.D.P., Y.C. and O.M. carried out the in situ synchrotron XCT–diffraction mapping. Z.N. and J.K. performed synthesis of Li6PS5Cl and powder X-ray diffraction characterization. Z.N. and S.D.P. performed the scanning electron microscopy experiment. G.L. and C.W.M. conducted the finite element analysis of current density distribution. Z.N., D.S.J., R.D.M., S.D.P., Y.C., C.G., B.L., P.A., D.M., G.O.H., T.J.M. and P.G.B. interpreted the data. Z.N. and P.G.B. wrote the manuscript with contributions and revisions from all authors. The project was supervised by T.J.M. and P.G.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8.
Rights and permissions
About this article
Cite this article
Ning, Z., Jolly, D.S., Li, G. et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021). https://doi.org/10.1038/s41563-021-00967-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-00967-8
This article is cited by
-
Effect of solid-electrolyte pellet density on failure of solid-state batteries
Nature Communications (2024)
-
Deciphering the critical role of interstitial volume in glassy sulfide superionic conductors
Nature Communications (2024)
-
Interfacial self-healing polymer electrolytes for long-cycle solid-state lithium-sulfur batteries
Nature Communications (2024)
-
From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments
Nano-Micro Letters (2024)
-
Boosting the interfacial superionic conduction of halide solid electrolytes for all-solid-state batteries
Nature Communications (2023)