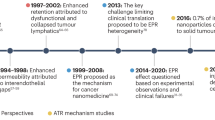
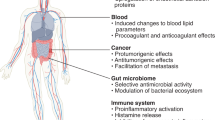
Abstract
Nanoparticle delivery to solid tumours over the past ten years has stagnated at a median of 0.7% of the injected dose. Varying nanoparticle designs and strategies have yielded only minor improvements. Here we discovered a dose threshold for improving nanoparticle tumour delivery: 1 trillion nanoparticles in mice. Doses above this threshold overwhelmed Kupffer cell uptake rates, nonlinearly decreased liver clearance, prolonged circulation and increased nanoparticle tumour delivery. This enabled up to 12% tumour delivery efficiency and delivery to 93% of cells in tumours, and also improved the therapeutic efficacy of Caelyx/Doxil. This threshold was robust across different nanoparticle types, tumour models and studies across ten years of the literature. Our results have implications for human translation and highlight a simple, but powerful, principle for designing nanoparticle cancer treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information files. The raw data that support the findings of this study are available from the corresponding author on reasonable request. Additional data from the meta-analysis of literature are available from the Cancer Nanomedicine Repository at http://inbs.med.utoronto.ca/cnr/.
Code availability
All code (used to run the simulation data in Supplementary Figs. 12 and 13) is available via GitHub at https://github.com/beeno/trillionParticlesODEs. All code for 3D image analysis is available via GitHub at https://github.com/BenKingston/nanoparticle_vessel_analysis.
References
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Rahman, Y. E., Cerny, E. A., Patel, K. R., Lau, E. H. & Wright, B. J. Differential uptake of liposomes varying in size and lipid composition by parenchymal and Kupffer cells of mouse liver. Life Sci. 31, 2061–2071 (1982).
Perrault, S. D., Walkey, C., Jennings, T., Fischer, H. C. & Chan, W. C. W. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9, 1909–1915 (2009).
Arnida, Janát-Amsbury, M. M., Ray, A., Peterson, C. M. & Ghandehari, H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur. J. Pharm. Biopharm. 77, 417–423 (2011).
Huang, X. et al. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 5, 5390–5399 (2011).
Juliano, R. L. & Stamp, D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 63, 651–658 (1975).
Albanese, A., Tang, P. S. & Chan, W. C. W. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 14, 1–16 (2012).
Tavares, A. J. et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl Acad. Sci. USA 114, E10871–E10880 (2017).
Wolfram, J. et al. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 7, 1–13 (2017).
Proffitt, R. T. et al. Liposoinal blockade of the reticuloendothelial system: improved tumor imaging with small unilamellar vesicles. Science 220, 502–505 (1983).
Cheng, Y.-H., He, C., Riviere, J. E., Monteiro-Riviere, N. A. & Lin, Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 14, 3075–3095 (2020).
Zhang, Y.-N., Poon, W., Tavares, A. J., McGilvray, I. D. & Chan, W. C. W. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Release 240, 332–348 (2016).
Liu, T., Choi, H., Zhou, R. & Chen, I. W. RES blockade: a strategy for boosting efficiency of nanoparticle drug. Nano Today 10, 11–21 (2015).
Sun, X. et al. Improved tumor uptake by optimizing liposome based RES blockade strategy. Theranostics 7, 319–328 (2017).
Drummond, D. C., Noble, C. O., Hayes, M. E., Park, J. W. & Kirpotin, D. B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 97, 4696–4740 (2008).
Tsoi, K. M. et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 15, 1212–1221 (2016).
Iio, M. & Wagner, H. N. Studies of the reticuloendothelial system (RES). I. Measurement of the phagocytic capacity of the RES in man and dog. J. Clin. Invest. 42, 417–426 (1963).
Allen, T. M. & Hansen, C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. BBA Biomembr. 1068, 133–141 (1991).
Laverman, P. et al. Preclinical and clinical evidence for disappearance of long-circulating characteristics of polyethylene glycol liposomes at low lipid dose. J. Pharmacol. Exp. Ther. 293, 996–1001 (2000).
Utkhede, D. R. & Tilcock, C. P. Effect of Lipid dose on the redistribution and blood pool clearance kinetics of PEG-modified technetium-labeled lipid vesicles. J. Liposome Res. 8, 381–390 (2008).
Kai, M. P. et al. Tumor presence induces global immune changes and enhances nanoparticle clearance. ACS Nano 10, 861–870 (2016).
Bouwens, L., Baekeland, M., de Zanger, R. & Wisse, E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology 6, 718–722 (1986).
Lee, S.-H., Starkey, P. M. & Gordon, S. Quantitative analysis of total macrophage content in adult mouse tissues: immunochemical studies with monoclonal antibody F4/80. J. Exp. Med. 161, 475–489 (1985).
Park, J.-K. et al. Cellular distribution of injected PLGA-nanoparticles in the liver. Nanomedicine 12, 1365–1374 (2016).
Poon, W. et al. Elimination pathways of nanoparticles. ACS Nano 13, 5785–5798 (2019).
Ishida, T. & Kiwada, H. Accelerated blood clearance (ABC) phenomenon upon repeated injection of PEGylated liposomes. Int. J. Pharm. 354, 56–62 (2008).
Sasmono, R. T. et al. A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101, 1155–1163 (2003).
Kolb-Bachofen, V., Schlepper-Schäfer, J. & Kolb, H. Receptor-mediated particle uptake by liver macrophages. Exp. Cell Res. 148, 173–182 (1983).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Canton, J. et al. Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages. Nat. Commun. 7, 11284 (2016).
Lazarovits, J. et al. Supervised learning and mass spectrometry predicts the in vivo fate of nanomaterials. ACS Nano 13, 8023–8034 (2019).
Gordon, S. Phagocytosis: an immunobiologic process. Immunity 44, 463–475 (2016).
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nat. Rev. Cancer 6, 583–592 (2006).
Sykes, E. A., Chen, J., Zheng, G. & Chan, W. C. W. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 8, 5696–5706 (2014).
Kingston, B. R., Syed, A. M., Ngai, J., Sindhwani, S. & Chan, W. C. W. Assessing micrometastases as a target for nanoparticles using 3D microscopy and machine learning. Proc. Natl Acad. Sci. USA 116, 14937–14946 (2019).
Syed, A. M. et al. Three-dimensional imaging of transparent tissues via metal nanoparticle labeling. J. Am. Chem. Soc. 139, 9961–9971 (2017).
Gabizon, A., Tzemach, D., Mak, L., Bronstein, M. & Horowitz, A. T. Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J. Drug Target. 10, 539–548 (2002).
Ohara, Y. et al. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int. J. Cancer 131, 2402–2410 (2012).
Charrois, G. J. R. & Allen, T. M. Multiple injections of pegylated liposomal doxorubicin: pharmacokinetics and therapeutic activity. J. Pharmacol. Exp. Ther. 306, 1058–1067 (2003).
Simberg, D. et al. Biomimetic amplification of nanoparticle homing to tumors. Proc. Natl. Acad. Sci. USA 104, 932–936 (2007).
Souhami, R. L., Patel, H. M. & Ryman, B. E. The effect of reticuloendothelial blockade on the blood clearance and tissue distribution of liposomes. Biochim. Biophys. Acta 674, 354–371 (1981).
Fernández-Urrusuno, R. et al. Effect of polymeric nanoparticle administration on the clearance activity of the mononuclear phagocyte system in mice. J. Biomed. Mater. Res. 31, 401–408 (1996).
Wagner, H. N. & Iio, M. Studies of the reticuloendothelial system (RES). III. Blockade of the RES in man. J. Clin. Invest. 43, 1525–1532 (1964).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134, 2139–2147 (2012).
Mcneil, S. E. Evaluation of nanomedicines: stick to the basics. Nat. Rev. Mater. 1, 16073 (2016).
Wilhelm, S., Tavares, A. J. & Chan, W. C. W. Reply to “Evaluation of nanomedicines: stick to the basics”. Nat. Rev. Mater. 1, 16074 (2016).
Autio, K. A. et al. Safety and eficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol. 4, 1344–1351 (2018).
Fujiwara, Y. et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 120, 475–480 (2019).
Hrkach, J. et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 4, 128ra39–128ra39 (2012).
Rangarajan, S. et al. AAV5–factor VIII gene transfer in severe hemophilia A. N. Engl. J. Med. 377, 2519–2530 (2017).
Sindhwani, S. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020).
Szebeni, J., Simberg, D., González-Fernández, Á., Barenholz, Y. & Dobrovolskaia, M. A. Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol. 13, 1100–1108 (2018).
La-beck, N. M. & Gabizon, A. A. Nanoparticle interactions with the immune system: clinical implications for liposome-based cancer chemotherapy. Front. Immunol. 8, 6–11 (2017).
Jensen, A. I. et al. Remote-loading of liposomes with manganese-52 and in vivo evaluation of the stabilities of 52Mn-DOTA and 64Cu-DOTA using radiolabelled liposomes and PET imaging. J. Control. Release 269, 100–109 (2018).
Pulaski, B. A. & Ostrand-Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. 39, 20.2.1–20.2.16 (2000).
Albanese, A., Tsoi, K. M. & Chan, W. C. W. Simultaneous quantification of cells and nanomaterials by inductive-coupled plasma techniques. J. Lab. Autom. 18, 99–104 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Adobe Photoshop 21.0.2 (Adobe, 2019).
Akima, H. A New Method of interpolation and smooth curve fitting based on local procedures. J. ACM 17, 589–602 (1970).
Sindhwani, S. et al. Three-dimensional optical mapping of nanoparticle distribution in intact tissues. ACS Nano 10, 5468–5478 (2016).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
FlowJo Software v.10.0.7 (Becton, Dickinson and Company, 2019).
GraphPad Prism v.8.4.0 (GraphPad Software).
Acknowledgements
We thank W. Jiang, W. Hou and T. Komal for help with experiments involving radioactivity, S. Lheureux for Caelyx and M. Ganguly, V. Bradaschia and K. Duffin in Pathology at The Centre for Phenogenomics for histology and blood biochemistry. We thank M. Egeblad for the c-fms-EGFP BALB/c breeder mice, and S. Grinstein for RAW264.7 cells. We thank L. Dunning and the Division of Comparative Medicine for animal husbandry. We thank J. Rothschild, C. E. Shin, S. Wilhelm, S. MacParland, J. Jonkman, S. Grinstein and K. Kataoka for discussions. We thank A. Malekjahani, B. Udugama, S. MacParland, M. Rajora, J. Ngai and S. Wilhelm for discussions with the manuscript revisions. We thank the Toronto Nanomedicine Fabrication Centre for use of the ICP-MS, the Nanoscale Biomedical Imaging Facility for use of the TEM and the Advanced Optical Microscopy Facility for guidance and use of the intravital microscope. This work was supported by the Canadian Cancer Society (grant numbers 502200 and 706286), the Canadian Institutes of Health Research (grant numbers PJT-148848 and FDN-159932), the Natural Sciences and Engineering Research Council of Canada (grant number 2015–06397), the Canada Research Chair Program (grant numbers 950–223924 and 950–232468), the Canada Foundation for Innovation (grant number 21765) and the Princess Margaret Cancer Foundation. B.O. thanks the Vanier Canada Graduate Scholarship, CIHR and the McLaughlin Centre for MD/PhD studentships, and the Ontario Graduate Scholarships, the Institute of Biomaterials and Biomedical Engineering, the University of Toronto School of Graduate Studies, the Donnelly Centre, the Frank Fletcher Memorial Fund, C. Yip and J. J. Ruffo for graduate fellowships. W.P. thanks the CIHR and OGS for graduate scholarships, and acknowledges fellowship support from C. Yip, B. and F. Milligan and the University of Toronto Faculty of Applied Science and Engineering. Y.-N.Z. thanks the NSERC, Wildcat Foundation, Ontario Graduate Scholarship, and Paul and Sally Wang fellowships. B.R.K. thanks NSERC, the Donnelly Centre, the Wildcat Fellows Program, the Royal Bank of Canada and Borealis AI for student fellowships and scholarships. A.J.T. thanks CIHR for the provision of a postdoctoral fellowship. M.S.V. thanks the Department of Defense Ovarian Cancer Research Program and the Terry Fox Research Institute for funding. J.C.-S. thanks the University of Toronto Faculty of Medicine for funding. P.M. thanks the Walter C. Sumner foundation for the fellowship.
Author information
Authors and Affiliations
Contributions
B.O., W.P., Y.-N.Z. and W.C.W.C. conceptualized the project. B.O., W.P., Y.-N.Z., Z.P.L., B.R.K., A.M.S., A.J.T., P.M. and J.C.-S. designed and performed the nanoparticle synthesis and biodistribution experiments. J.C., M.S.V. and B.O. designed and performed the radioactive liposome validation biodistribution experiments. B.O., W.P., Y.-N.Z. and Z.P.L. designed and performed the delivery enhancer experiments. B.R.K., A.M.S. and P.M. designed and performed the 3D tissue microscopy experiments. Y.Z. designed and performed the protein corona analysis experiments. G.Z. and W.C.W.C. acquired funding for this project. B.O. and W.C.W.C. wrote the initial manuscript draft. All authors contributed to reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.O., W.P., Y.-N.Z., Z.P.L. and W.C.W.C. declare patents pending on the delivery enhancer technique in the United States (63/017,322) and Canada (3,079,765).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Tables 1–3, video captions, methods and references.
Supplementary Video 1
Live intravital imaging of Cy3 gold nanoparticle uptake in Kupffer cells in a mouse administered with a low dose.
Supplementary Video 2
Live intravital imaging of Cy3 gold nanoparticle uptake in Kupffer cells in a mouse administered with a high dose.
Supplementary Video 3
Live intravital imaging of Cy5 gold nanoparticle uptake in Kupffer cells in a mouse administered with a high dose.
Rights and permissions
About this article
Cite this article
Ouyang, B., Poon, W., Zhang, YN. et al. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 19, 1362–1371 (2020). https://doi.org/10.1038/s41563-020-0755-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0755-z
This article is cited by
-
SiO2–alginate–melittin nano-conjugates suppress the proliferation of ovarian cancer cells: a controlled release approach leveraging alginate lyase
Cancer Nanotechnology (2024)
-
Spatially selective delivery of living magnetic microrobots through torque-focusing
Nature Communications (2024)
-
Reexamining the effects of drug loading on the in vivo performance of PEGylated liposomal doxorubicin
Acta Pharmacologica Sinica (2024)
-
The mechanisms of nanoparticle delivery to solid tumours
Nature Reviews Bioengineering (2024)
-
Molecular Mechanisms of Intracellular Delivery of Nanoparticles Monitored by an Enzyme-Induced Proximity Labeling
Nano-Micro Letters (2024)