Abstract
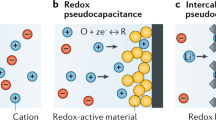
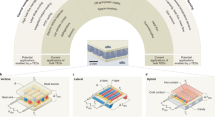
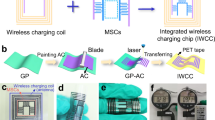
Electrochemical capacitors can store electrical energy harvested from intermittent sources and deliver energy quickly, but their energy density must be increased if they are to efficiently power flexible and wearable electronics, as well as larger equipment. This Review summarizes progress in the field of materials for electrochemical capacitors over the past decade as well as outlines key perspectives for future research. We describe electrical double-layer capacitors based on high-surface-area carbons, pseudocapacitive materials such as oxides and the two-dimensional inorganic compounds known as MXenes, and emerging microdevices for the Internet of Things. We show that new nanostructured electrode materials and matching electrolytes are required to maximize the amount of energy and speed of delivery, and different manufacturing methods will be needed to meet the requirements of the future generation of electronic devices. Scientifically justified metrics for testing, comparison and optimization of various kinds of electrochemical capacitors are provided and explained.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



O. Fontaine



Similar content being viewed by others
References
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Delmas, C. Sodium and sodium-ion batteries: 50 years of research. Adv. Energy Mater. 8, 1703137 (2018).
Li, M., Lu, J., Chen, Z. & Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 30, 1800561 (2018).
Palacin, M. R. & de Guibert, A. Why do batteries fail? Science 351, 1253292 (2016).
Augustyn, V., Simon, P. & Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 7, 1597–1614 (2014).
Liu, C. F., Liu, Y. C., Yi, T. Y. & Hu, C. C. Carbon materials for high-voltage supercapacitors. Carbon 145, 529–548 (2019).
Wang, F. X. et al. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem. Soc. Rev. 46, 6816–6854 (2017).
Wang, Y. G., Song, Y. F. & Xia, Y. Y. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 45, 5925–5950 (2016).
Wu, H. et al. Graphene based architectures for electrochemical capacitors. Energy Storage Mater. 5, 8–32 (2016).
Lin, Z. et al. Materials for supercapacitors: when Li-ion battery power is not enough. Mater. Today 21, 419–436 (2018).
Noori, A., El-Kady, M. F., Rahmanifar, M. S., Kaner, R. B. & Mousavi, M. F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 48, 1272–1341 (2019).
Salanne, M. et al. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 1, 16070 (2016).
Miller, J. R., Outlaw, R. A. & Holloway, B. C. Graphene double-layer capacitor with ac line-filtering performance. Science 329, 1637–1639 (2010).
Horn, M., MacLeod, J., Liu, M., Webb, J. & Motta, N. Supercapacitors: a new source of power for electric cars? Econ. Anal. Policy 61, 93–103 (2019).
Ultracapacitor modules. Maxwell Technologies https://www.maxwell.com/products/ultracapacitors/modules (accessed May 2020).
LS Ultracapacitor https://www.ultracapacitor.co.kr:8001/ (accessed May 2020).
Skeleton Technologies https://www.skeletontech.com/ (accessed May 2020).
Gogotsi, Y. & Simon, P. True performance metrics in electrochemical energy storage. Science 334, 917–918 (2011).
Mathis, T. S. et al. Energy storage data reporting in perspective — guidelines for interpreting the performance of electrochemical energy storage systems. Adv. Energy Mater. 9, 1902007 (2019).
Conway, B. Electrochemical Supercapacitors; Scientific Fundamentals and Technological Applications (Springer, 1999).
Shao, H., Wu, Y.-C., Lin, Z., Taberna, P.-L. & Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 49, 3005–3039 (2020).
Lin, T. Q. et al. Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 350, 1508–1513 (2015).
Beguin, F., Presser, V., Balducci, A. & Frackowiak, E. Carbons and electrolytes for advanced supercapacitors. Adv. Mater. 26, 2219–2251 (2014).
Zhong, C. et al. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 44, 7484–7539 (2015).
Miller, J. R. & Butler, S. M. Electrical characteristics of large state-of-the-art electrochemical capacitors. Electrochim. Acta 307, 564–572 (2019).
Xiong, G., Kundu, A. & Fisher, S. T. Thermal Effects in Supercapacitors (Springer, 2015).
Prehal, C. et al. Quantification of ion confinement and desolvation in nanoporous carbon supercapacitors with modelling and in situ X-ray scattering. Nat. Energy 2, 16215 (2017).
Forse, A. C. et al. Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy. Nat. Energy 2, 16216 (2017).
Chmiola, J. et al. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313, 1760–1763 (2006).
Forse, A. C., Merlet, C., Griffin, J. M. & Grey, C. P. New perspectives on the charging mechanisms of supercapacitors. J. Am. Chem. Soc. 138, 5731–5744 (2016).
Griffin, J. M. et al. In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors. Nat. Mater. 14, 812–819 (2015).
Deschamps, M. et al. Exploring electrolyte organization in supercapacitor electrodes with solid-state NMR. Nat. Mater. 12, 351–358 (2013).
Boukhalfa, S. et al. In situ small angle neutron scattering revealing ion sorption in microporous carbon electrical double layer capacitors. ACS Nano 8, 2495–2503 (2014).
Batisse, N. & Raymundo-Pinero, E. Pulsed electrochemical mass spectrometry for operando tracking of interfacial processes in small-time-constant electrochemical devices such as supercapacitors. ACS Appl. Mater. Interfaces 9, 41224–41232 (2017).
Kim, J. et al. Nondisruptive in situ Raman analysis for gas evolution in commercial supercapacitor cells. Electrochim. Acta 219, 447–452 (2016).
Richey, F. W., Dyatkin, B., Gogotsi, Y. & Elabd, Y. A. Ion dynamics in porous carbon electrodes in supercapacitors using in situ infrared spectroelectrochemistry. J. Am. Chem. Soc. 135, 12818–12826 (2013).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nat. Mater. 13, 387–393 (2014).
Pean, C. et al. On the dynamics of charging in nanoporous carbon-based supercapacitors. ACS Nano 8, 1576–1583 (2014).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 11, 306–310 (2012).
Kondrat, S. & Kornyshev, A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 23, 022201 (2011).
Bi, S. et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 19, 552–558 (2020).
Tsai, W. Y., Taberna, P. L. & Simon, P. Electrochemical quartz crystal microbalance (EQCM) study of ion dynamics in nanoporous carbons. J. Am. Chem. Soc. 136, 8722–8728 (2014).
Shpigel, N., Levi, M. D., Sigalov, S., Daikhin, L. & Aurbach, D. In situ real-time mechanical and morphological characterization of electrodes for electrochemical energy storage and conversion by electrochemical quartz crystal microbalance with dissipation monitoring. Acc. Chem. Res. 51, 69–79 (2018).
Shpigel, N. et al. In situ hydrodynamic spectroscopy for structure characterization of porous energy storage electrodes. Nat. Mater. 15, 570–575 (2016).
Le, T. et al. Unveiling the ionic exchange mechanisms in vertically-oriented graphene nanosheet supercapacitor electrodes with electrochemical quartz crystal microbalance and ac-electrogravimetry. Electrochem. Commun. 93, 5–9 (2018).
Umeda, K., Kobayashi, K., Minato, T. & Yamada, H. Atomic-scale three-dimensional local solvation structures of ionic liquids. J. Phys. Chem. Lett. 11, 1343–1348 (2020).
Tsai, W.-Y. et al. Hysteretic order–disorder transitions of ionic liquid double layer structure on graphite. Nano Energy 60, 886–893 (2019).
Ye, J. L. et al. Charge storage mechanisms of single-layer graphene in ionic liquid. J. Am. Chem. Soc. 141, 16559–16563 (2019).
Mao, X. et al. Self-assembled nanostructures in ionic liquids facilitate charge storage at electrified interfaces. Nat. Mater. 18, 1350–1357 (2019).
Jackel, N., Simon, P., Gogotsi, Y. & Presser, V. Increase in capacitance by subnanometer pores in carbon. ACS Energy Lett. 1, 1262–1265 (2016).
Yan, R. Y., Antonietti, M. & Oschatz, M. Toward the experimental understanding of the energy storage mechanism and ion dynamics in ionic liquid based supercapacitors. Adv. Energy Mater. 8, 1800026 (2018).
Antonietti, M., Chen, X. D., Yan, R. Y. & Oschatz, M. Storing electricity as chemical energy: beyond traditional electrochemistry and double-layer compression. Energy Environ. Sci. 11, 3069–3074 (2018).
Futamura, R. et al. Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores. Nat. Mater. 16, 1225–1232 (2017).
Bazant, M. Z., Storey, B. D. & Kornyshev, A. A. Double layer in ionic liquids: overscreening versus crowding. Phys. Rev. Lett. 106, 046102 (2011).
Liu, Y. M., Merlet, C. & Smit, B. Carbons with regular pore geometry yield fundamental insights into supercapacitor charge storage. ACS Cent. Sci. 5, 1813–1823 (2019).
Redondo, E. et al. Outstanding room-temperature capacitance of biomass-derived microporous carbons in ionic liquid electrolyte. Electrochem. Commun. 79, 5–8 (2017).
Miller, J. R. & Burke, A. F. Electrochemical capacitors: challenges and opportunities for real-world applications. Electrochem. Soc. Interface 8, 53–57 (2008).
Pognon, G., Brousse, T., Demarconnay, L. & Belanger, D. Performance and stability of electrochemical capacitor based on anthraquinone modified activated carbon. J. Power Sources 196, 4117–4122 (2011).
Assresahegn, B. D., Brousse, T. & Belanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 92, 362–381 (2015).
Nomura, K., Nishihara, H., Kobayashi, N., Asada, T. & Kyotani, T. 4.4 V supercapacitors based on super-stable mesoporous carbon sheet made of edge-free graphene walls. Energy Environ. Sci. 12, 1542–1549 (2019).
Antonietti, M. & Oschatz, M. The concept of ‘noble, heteroatom-doped carbons,’ their directed synthesis by electronic band control of carbonization, and applications in catalysis and energy materials. Adv. Mater. 30, 1706836 (2018).
Schutter, C., Passerini, S., Korth, M. & Balducci, A. Cyano ester as solvent for high voltage electrochemical double layer capacitors. Electrochim. Acta 224, 278–284 (2017).
Krummacher, J., Schütter, C., Hess, L. H. & Balducci, A. Non-aqueous electrolytes for electrochemical capacitors. Curr. Opin. Electrochem. 9, 64–69 (2018).
Xie, H. J., Gelinas, B. & Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 66, 42–45 (2016).
Mourad, E. et al. Biredox ionic liquids with solid-like redox density in the liquid state for high-energy supercapacitors. Nat. Mater. 16, 446–453 (2017).
Birkl, C. R., Roberts, M. R., McTurk, E., Bruce, P. G. & Howey, D. A. Degradation diagnostics for lithium ion cells. J. Power Sources 341, 373–386 (2017).
Singh, N. et al. Artificial SEI transplantation: a pathway to enabling lithium metal cycling in water-containing electrolyte. ACS Appl. Energy Mater. 2, 8912–8918 (2019).
Son, S. B. et al. An artificial interphase enables reversible magnesium chemistry in carbonate electrolytes. Nat. Chem. 10, 532–539 (2018).
Brandon, E. J., West, W. C., Smart, M. C., Whitcanack, L. D. & Plett, G. A. Extending the low temperature operational limit of double-layer capacitors. J. Power Sources 170, 225–232 (2007).
Kunze, M. et al. Mixtures of ionic liquids for low temperature electrolytes. Electrochim. Acta 82, 69–74 (2012).
Tsai, W. Y. et al. Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from −50 to 80 °C. Nano Energy 2, 403–411 (2013).
Premathilake, D. et al. Fast response, carbon-black-coated, vertically-oriented graphene electric double layer capacitors. J. Electrochem. Soc. 165, A924–A931 (2018).
Miller, J. R. & Outlaw, R. A. Vertically-oriented graphene electric double layer capacitor designs. J. Electrochem. Soc. 162, A5077–A5082 (2015).
Choi, C. et al. Achieving high energy density and high power density with pseudocapacitive materials. Nat. Rev. Mater. 5, 5–19 (2020).
Lee, H. Y. & Goodenough, J. B. Supercapacitor behavior with KCl electrolyte. J. Solid State Chem. 144, 220–223 (1999).
Brousse, T., Belanger, D. & Long, J. W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 162, A5185–A5189 (2015).
Simon, P., Gogotsi, Y. & Dunn, B. Where do batteries end and supercapacitors begin? Science 343, 1210–1211 (2014).
Lukatskaya, M. R., Dunn, B. & Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 7, 12647 (2016).
Wang, J., Polleux, J., Lim, J. & Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 111, 14925–14931 (2007).
Ko, J. S., Sassin, M. B., Rolison, D. R. & Long, J. W. Deconvolving double-layer, pseudocapacitance, and battery-like charge-storage mechanisms in nanoscale LiMn2O4 at 3D carbon architectures. Electrochim. Acta 275, 225–235 (2018).
Gibson, A. J. & Donne, S. W. A step potential electrochemical spectroscopy (SPECS) investigation of anodically electrodeposited thin films of manganese dioxide. J. Power Sources 359, 520–528 (2017).
Shao, H., Lin, Z., Xu, K., Taberna, P.-L. & Simon, P. Electrochemical study of pseudocapacitive behavior of Ti3C2Tx MXene material in aqueous electrolytes. Energy Storage Mater. 18, 456–461 (2019).
Costentin, C. & Saveant, J. M. Energy storage: pseudocapacitance in prospect. Chem. Sci. 10, 5656–5666 (2019).
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Wang, X. et al. Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy 4, 241–248 (2019).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Lukatskaya, M. R. et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2, 17105 (2017).
Ghidiu, M., Lukatskaya, M. R., Zhao, M. Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Augustyn, V. et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 12, 518–522 (2013).
Girard, H. L., Dunn, B. & Pilon, L. Simulations and interpretation of three-electrode cyclic voltammograms of pseudocapacitive electrodes. Electrochim. Acta 211, 420–429 (2016).
Conway, B. E. Two-dimensional and quasi two-dimensional isotherms for Li intercalation and UPD processes at surfaces. Electrochim. Acta 38, 1249–1258 (1993).
Li, Y. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 19, 894–899 (2020).
Naoi, K. et al. Ultrafast charge–discharge characteristics of a nanosized core–shell structured LiFePO4 material for hybrid supercapacitor applications. Energy Environ. Sci. 9, 2143–2151 (2016).
Amisse, R. et al. Singular structural and electrochemical properties in highly defective LiFePO4 powders. Chem. Mater. 27, 4261–4273 (2015).
Okubo, M. et al. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 129, 7444–7452 (2007).
Kim, H. S. et al. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3−x. Nat. Mater. 16, 454–460 (2017).
Wang, R. C. et al. Operando atomic force microscopy reveals mechanics of structural water driven battery-to-pseudocapacitor transition. ACS Nano 12, 6032–6039 (2018).
Mitchell, J. B. et al. Confined interlayer water promotes structural stability for high-rate electrochemical proton intercalation in tungsten oxide hydrates. ACS Energy Lett. 4, 2805–2812 (2019).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Han, P. X. et al. Lithium ion capacitors in organic electrolyte system: scientific problems, material development, and key technologies. Adv. Energy Mater. 8, 1801243 (2018).
Jezowski, P. et al. Safe and recyclable lithium-ion capacitors using sacrificial organic lithium salt. Nat. Mater. 17, 167–173 (2018).
Takami, N. et al. High-energy, fast-charging, long-life lithium-ion batteries using TiNb2O7 anodes for automotive applications. J. Power Sources 396, 429–436 (2018).
Whitmore, A., Agarwal, A. & Xu, L. D. The Internet of Things — a survey of topics and trends. Inform. Syst. Front. 17, 261–274 (2015).
Lethien, C., Le Bideau, J. & Brousse, T. Challenges and prospects of 3D micro-supercapacitors for powering the internet of things. Energy Environ. Sci. 12, 96–115 (2019).
Huang, P. et al. On-chip and freestanding elastic carbon films for micro-supercapacitors. Science 351, 691–695 (2016).
Kyeremateng, N. A., Brousse, T. & Pech, D. Microsupercapacitors as miniaturized energy-storage components for on-chip electronics. Nat. Nanotechnol. 12, 7–15 (2017).
Negre, L., Daffos, B., Turq, V., Taberna, P. L. & Simon, P. Ionogel-based solid-state supercapacitor operating over a wide range of temperature. Electrochim. Acta 206, 490–495 (2016).
Brachet, M., Brousse, T. & Le Bideau, J. All solid-state symmetrical activated carbon electrochemical double layer capacitors designed with ionogel electrolyte. ECS Electrochem. Lett. 3, A112–A115 (2014).
Sumboja, A. et al. Electrochemical energy storage devices for wearable technology: a rationale for materials selection and cell design. Chem. Soc. Rev. 47, 5919–5945 (2018).
El-Kady, M. F., Strong, V., Dubin, S. & Kaner, R. B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 335, 1326–1330 (2012).
Lin, J. et al. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 5, 5714 (2014).
El-Kady, M. F. et al. Engineering three-dimensional hybrid supercapacitors and microsupercapacitors for high-performance integrated energy storage. Proc. Natl Acad. Sci. USA 112, 4233–4238 (2015).
Ye, L. H. et al. Highly efficient materials assembly via electrophoretic deposition for electrochemical energy conversion and storage devices. Adv. Energy Mater. 6, 1502018 (2016).
Ferris, A., Garbarino, S., Guay, D. & Pech, D. 3D RuO2 microsupercapacitors with remarkable areal energy. Adv. Mater. 27, 6625–6629 (2015).
Zhang, Y. Z. et al. Printed supercapacitors: materials, printing and applications. Chem. Soc. Rev. 48, 3229–3264 (2019).
Jost, K., Dion, G. & Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2, 10776–10787 (2014).
Garcia-Torres, J., Roberts, A. J., Slade, R. C. T. & Crean, C. One-step wet-spinning process of CB/CNT/MnO2 nanotubes hybrid flexible fibres as electrodes for wearable supercapacitors. Electrochim. Acta 296, 481–490 (2019).
Quain, E. et al. Direct writing of additive-free MXene-in-water ink for electronics and energy storage. Adv. Mater. Technol. 4, 1800256 (2019).
Acknowledgements
P.S. acknowledges H. Shao, P. Rozier and C. Merlet for help with the figures, as well as the Agence Nationale de la Recherche (Labex Store-Ex) and Institut Universitaire de France for support. Y.G.’s research on capacitive energy storage was primarily supported through the Fluid Interface Reactions, Structures, and Transport (FIRST) Center, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, and Office of Basic Energy Sciences.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to the planning and writing of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Simon, P., Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 19, 1151–1163 (2020). https://doi.org/10.1038/s41563-020-0747-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0747-z
This article is cited by
-
Nanofeather ruthenium nitride electrodes for electrochemical capacitors
Nature Materials (2024)
-
Synthesis of thermosets from maleimidobenzoxazines and tetrafunctional thiols and their thermal and mechanical properties
Polymer Journal (2024)
-
Optimizing the fabrication of Co compounds coating on modified carbon cloth with enhanced pseudocapacitive performance and cyclic stability
Journal of Solid State Electrochemistry (2024)
-
Understanding the role of precursor concentration in the hydrothermal synthesis of nickel phosphate hydrate for supercapacitors
Journal of Materials Science: Materials in Electronics (2024)
-
Coupling of Adhesion and Anti-Freezing Properties in Hydrogel Electrolytes for Low-Temperature Aqueous-Based Hybrid Capacitors
Nano-Micro Letters (2024)