Abstract
The fast penetration of electrification in rural areas calls for the development of competitive decentralized approaches. A promising solution is represented by low-cost and compact integrated solar flow batteries; however, obtaining high energy conversion performance and long device lifetime simultaneously in these systems has been challenging. Here, we use high-efficiency perovskite/silicon tandem solar cells and redox flow batteries based on robust BTMAP-Vi/NMe-TEMPO redox couples to realize a high-performance and stable solar flow battery device. Numerical analysis methods enable the rational design of both components, achieving an optimal voltage match. These efforts led to a solar-to-output electricity efficiency of 20.1% for solar flow batteries, as well as improved device lifetime, solar power conversion utilization ratio and capacity utilization rate. The conceptual design strategy presented here also suggests general future optimization approaches for integrated solar energy conversion and storage systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper. The remaining data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Eke, K. P. Emerging considerations of rural electrification infrastructure development in Africa. In 2017 IEEE PES Power Africa Conference 138–142 (IEEE, 2017).
Cook, P. Infrastructure, rural electrification and development. Energy Sustain. Dev. 15, 304–313 (2011).
Wamukonya, N. Solar home system electrification as a viable technology option for Africa’s development. Energy Policy 35, 6–14 (2007).
Halder, P. K. Potential and economic feasibility of solar home systems implementation in Bangladesh. Renew. Sustain. Energy Rev. 65, 568–576 (2016).
Charles, R. G., Davies, M. L., Douglas, P., Hallin, I. L. & Mabbett, I. Sustainable energy storage for solar home systems in rural Sub-Saharan Africa—a comparative examination of lifecycle aspects of battery technologies for circular economy, with emphasis on the South African context. Energy 166, 1207–1215 (2019).
Gurung, A. & Qiao, Q. Q. Solar charging batteries: advances, challenges, and opportunities. Joule 2, 1217–1230 (2018).
Schmidt, D., Hager, M. D. & Schubert, U. S. Photo-rechargeable electric energy storage systems. Adv. Energy Mater. 6, 1500369 (2015).
Yu, M. et al. Solar-powered electrochemical energy storage: an alternative to solar fuels. J. Mater. Chem. A 4, 2766–2782 (2016).
Wedege, K., Bae, D., Smith, W. A., Mendes, A. & Bentien, A. Solar redox flow batteries with organic redox couples in aqueous electrolytes: a minireview. J. Phys. Chem. C 122, 25729–25740 (2018).
Wedege, K. et al. Unbiased, complete solar charging of a neutral flow battery by a single Si photocathode. RSC Adv. 8, 6331–6340 (2018).
Li, W., Fu, H.-C., Zhao, Y., He, J.-H. & Jin, S. 14.1% efficient monolithically integrated solar flow battery. Chem 4, 2644–2657 (2018).
Li, W. J. et al. A long lifetime aqueous organic solar flow battery. Adv. Energy Mater. 9, 1900918 (2019).
Yu, M. Z. et al. Aqueous lithium-iodine solar flow battery for the simultaneous conversion and storage of solar energy. J. Am. Chem. Soc. 137, 8332–8335 (2015).
Liao, S. et al. Integrating a dual-silicon photoelectrochemical cell into a redox flow battery for unassisted photocharging. Nat. Commun. 7, 11474–11478 (2016).
Li, W. et al. Integrated photoelectrochemical solar energy conversion and organic redox flow battery devices. Angew. Chem. Int. Ed. 55, 13104–13108 (2016).
Wedege, K., Azevedo, J., Khataee, A., Bentien, A. & Mendes, A. Direct solar charging of an organic-inorganic, stable, and aqueous alkaline redox flow battery with a hematite photoanode. Angew. Chem. Int. Ed. 55, 7142–7147 (2016).
Cheng, Q. et al. Photorechargeable high voltage redox battery enabled by Ta3N5 and GaN/Si dual-photoelectrode. Adv. Mater. 351, 1700312–1700318 (2017).
McKone, J. R., DiSalvo, F. J. & Abruna, H. D. Solar energy conversion, storage, and release using an integrated solar-driven redox flow battery. J. Mater. Chem. A 5, 5362–5372 (2017).
Zhou, Y. et al. Efficient solar energy harvesting and storage through a robust photocatalyst driving reversible redox reactions. Adv. Mater. 103, 1802294–1802297 (2018).
Hu, S. et al. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 344, 1005–1009 (2014).
Khan, M. A. et al. Importance of oxygen measurements during photoelectrochemical water-splitting reactions. ACS Energy Lett. 4, 2712–2718 (2019).
Liu, T., Wei, X., Nie, Z., Sprenkle, V. & Wang, W. A total organic aqueous redox flow battery employing a low cost and sustainable methyl viologen anolyte and 4-HO-TEMPO catholyte. Adv. Energy Mater. 6, 1501449 (2015).
Liu, Y. et al. A long lifetime all-organic aqueous flow battery utilizing TMAP-TEMPO radical. Chem 5, 1861–1870 (2019).
Hu, S. et al. Thin-film materials for the protection of semiconducting photoelectrodes in solar-fuel generators. J. Phys. Chem. C 119, 24201–24228 (2015).
Bae, D., Seger, B., Vesborg, P. C. K., Hansen, O. & Chorkendorff, I. Strategies for stable water splitting via protected photoelectrodes. Chem. Soc. Rev. 46, 1933–1954 (2017).
Beh, E. S. et al. A neutral pH aqueous organic-organometallic redox flow battery with extremely high capacity retention. ACS Energy Lett. 2, 639–644 (2017).
Green, M. A. et al. Solar cell efficiency tables (version 54). Prog. Photovolt. 27, 565–575 (2019).
Seger, B. et al. Using TiO2 as a conductive protective layer for photocathodic H2 evolution. J. Am. Chem. Soc. 135, 1057–1064 (2013).
Shaner, M. R., Hu, S., Sun, K. & Lewis, N. S. Stabilization of Si microwire arrays for solar-driven H2O oxidation to O2(g) in 1.0 M KOH(aq) using conformal coatings of amorphous TiO2. Energy Environ. Sci. 8, 203–207 (2015).
Zheng, J. H. et al. Large area efficient interface layer free monolithic perovskite/homo-junction-silicon tandem solar cell with over 20% efficiency. Energy Environ. Sci. 11, 2432–2443 (2018).
Zheng, J. H. et al. 21.8% efficient monolithic perovskite/homo-junction-silicon tandem solar cell on 16 cm(2). ACS Energy Lett. 3, 2299–2300 (2018).
Bush, K. A. et al. 23.6%-efficient monolithic perovskite/silicon tandem solar cells with improved stability. Nat. Energy 2, 17009 (2017).
Correa-Baena, J. P. et al. Promises and challenges of perovskite solar cells. Science 358, 739–744 (2017).
Yu, Z. S., Leilaeioun, M. & Holman, Z. Selecting tandem partners for silicon solar cells. Nat. Energy 1, 16137 (2016).
Ji, Y. et al. A phosphonate-functionalized quinone redox flow battery at near-neutral pH with record capacity retention rate. Adv. Energy Mater. 9, 1900039 (2019).
DeBruler, C. et al. Designer two-electron storage viologen anolyte materials for neutral aqueous organic redox flow batteries. Chem 3, 961–978 (2017).
Park, M., Ryu, J., Wang, W. & Cho, J. Material design and engineering of next-generation flow-battery technologies. Nat. Rev. Mater 2, 16080–16018 (2016).
Hollas, A. et al. A biomimetic high-capacity phenazine-based anolyte for aqueous organic redox flow batteries. Nat. Energy 3, 508–514 (2018).
Luo, J. A., Hu, B., Hu, M. W., Zhao, Y. & Liu, T. L. Status and prospects of organic redox flow batteries toward sustainable energy storage. ACS Energy Lett. 4, 2220–2240 (2019).
Weber, A. Z. et al. Redox flow batteries: a review. J. Appl. Electrochem. 41, 1137–1164 (2011).
Darling, R. M., Gallagher, K. G., Kowalski, J. A., Ha, S. & Brushett, F. R. Pathways to low-cost electrochemical energy storage: a comparison of aqueous and nonaqueous flow batteries. Energy Environ. Sci. 7, 3459–3477 (2014).
Hu, B., DeBruler, C., Rhodes, Z. & Liu, T. L. Long-cycling aqueous organic redox flow battery (AORFB) toward sustainable and safe energy storage. J. Am. Chem. Soc. 139, 1207–1214 (2017).
Goulet, M. A. & Aziz, M. J. Flow battery molecular reactant stability determined by symmetric cell cycling methods. J. Electrochem. Soc. 165, A1466–A1477 (2018).
Acknowledgements
This research is supported by the King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) under award no. OSR-2017-CRG6-3453.02 to both J.-H.H. and S.J. The Australian Centre for Advanced Photovoltaics (ACAP) encompasses the Australian-based activities of the Australia–US Institute for Advanced Photovoltaics (AUSIAPV) and is supported by the Australian Renewable Energy Agency (ARENA). J.Z. and A.H.-B. thank ARENA for support via project 2014 RND075. T.L.L., B.H. and M.H. acknowledge the US National Science Foundation (CAREER Award, grant no. 1847674) and Utah State University faculty start-up fund for support. B.H. and M.H. are grateful for China Scholarship Council (CSC) Abroad Studying Fellowships to support their PhD study at Utah State University. We thank Z. Wu for help with FcNCl synthesis, and D. Roberts and X. Liu for help with NMR.
Author information
Authors and Affiliations
Contributions
W.L. and S.J. designed the experiments. W.L. fabricated the SFB devices and carried out the electrochemical measurements with the help of H.-C.F. and A.V. W.L. and Y.Z. built the customized control device for SFB characterization. J.Z. and A.H.-B. fabricated the perovskite/silicon tandem solar cells. B.H., M.H. and T.L.L. synthesized the NMe-TEMPO and BTMAP-Vi redox couples. W.L. and S.J. wrote the manuscript and all authors commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–23, Notes 1–3 and Tables 1 and 2.
Source data
Source Data Fig. 1
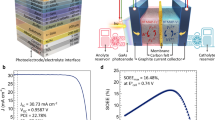
J-V performance of perovskite/silicon solar cell and photoelectrode.
Source Data Fig. 2
Numerical calculation of SOEE.
Source Data Fig. 3
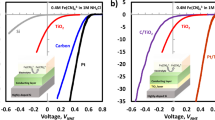
Electrochemical characterization of redox couples and RFBs.
Source Data Fig. 4
Cycling performance of integrated SFB.
Rights and permissions
About this article
Cite this article
Li, W., Zheng, J., Hu, B. et al. High-performance solar flow battery powered by a perovskite/silicon tandem solar cell. Nat. Mater. 19, 1326–1331 (2020). https://doi.org/10.1038/s41563-020-0720-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0720-x
This article is cited by
-
Potential window alignment regulating ion transfer in faradaic junctions for efficient photoelectrocatalysis
Nature Communications (2023)
-
Next-generation applications for integrated perovskite solar cells
Communications Materials (2023)
-
Polycrystalline silicon tunnelling recombination layers for high-efficiency perovskite/tunnel oxide passivating contact tandem solar cells
Nature Energy (2023)
-
Emerging chemistries and molecular designs for flow batteries
Nature Reviews Chemistry (2022)
-
Photovoltage memory effect in a portable Faradaic junction solar rechargeable device
Nature Communications (2022)