Abstract
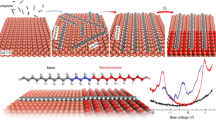
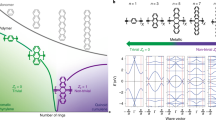
Two-dimensional materials with high charge carrier mobility and tunable band gaps have attracted intense research effort for their potential use in nanoelectronics. Two-dimensional π-conjugated polymers constitute a promising subclass because the band structure can be manipulated by varying the molecular building blocks while preserving key features such as Dirac cones and high charge mobility. The major barriers to the application of two-dimensional π-conjugated polymers have been the small domain size and high defect density attained in the syntheses explored so far. Here, we demonstrate the fabrication of mesoscale ordered two-dimensional π-conjugated polymer kagome lattices with semiconducting properties, Dirac cone structures and flat bands on Au(111). This material has been obtained by combining a rigid azatriangulene precursor and a hot dosing approach, which favours molecular diffusion and eliminates voids in the network. These results open opportunities for the synthesis of two-dimensional π-conjugated polymer Dirac cone materials and their integration into devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary information, while DFT calculations can be found at the following web repositor: https://doi.org/10.17172/NOMAD/2020.04.05-1. Extra data are available from the authors upon request.
Code availability
The codes and algorithms used for the statistical analysis of the STM images are available from the following web repository: https://github.com/lvbesteiro/STM-Minimum_Spanning_Tree.
References
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004).
Perepichka, D. F. & Rosei, F. Extending polymer conjugation into the second dimension. Science 323, 216–217 (2009).
Asano, K. & Hotta, C. Designing Dirac points in two-dimensional lattices. Phys. Rev. B 83, 245125 (2011).
Wang, J., Deng, S., Liu, Z. & Liu, Z. The rare two-dimensional materials with Dirac cones. Natl Sci. Rev. 2, 22–39 (2015).
Thomas, S. et al. Electronic structure of two-dimensional π-conjugated covalent organic frameworks. Chem. Mater. 31, 3051–3065 (2019).
Jing, Y. & Heine, T. Two-dimensional kagome lattices made of hetero triangulenes are Dirac semimetals or single-band semiconductors. J. Am. Chem. Soc. 141, 743–747 (2019).
Barreteau, C., Ducastelle, F. & Mallah, T. A bird’s eye view on the flat and conic band world of the honeycomb and Kagome lattices: towards an understanding of 2D metal-organic frameworks electronic structure. J. Phys. Condens. Matter 29, 465302 (2017).
Liu, Z., Liu, F. & Wu, Y.-S. Exotic electronic states in the world of flat bands: from theory to material. Chin. Phys. B 23, 077308 (2014).
Zhong, C., Xie, Y., Chen, Y. & Zhang, S. Coexistence of flat bands and Dirac bands in a carbon-Kagome-lattice family. Carbon 99, 65–70 (2016).
Adjizian, J.-J. et al. Dirac cones in two-dimensional conjugated polymer networks. Nat. Commun. 5, 5842 (2014).
Gutzler, R. & Perepichka, D. F. π-Electron conjugation in two dimensions. J. Am. Chem. Soc. 135, 16585–16594 (2013).
Gutzler, R. Band-structure engineering in conjugated 2D polymers. Phys. Chem. Chem. Phys. 18, 29092–29100 (2016).
Zhao, A. & Shen, S.-Q. Quantum anomalous Hall effect in a flat band ferromagnet. Phys. Rev. B 85, 085209 (2012).
Kopnin, N. B., Heikkilä, T. T. & Volovik, G. E. High-temperature surface superconductivity in topological flat-band systems. Phys. Rev. B 83, 220503 (2011).
Julku, A., Peotta, S., Vanhala, T. I., Kim, D.-H. & Törmä, P. Geometric origin of superfluidity in the Lieb-lattice flat band. Phys. Rev. Lett. 117, 045303 (2016).
Lackinger, M. Surface-assisted Ullmann coupling. Chem. Commun. 53, 7872–7885 (2017).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Lafferentz, L. et al. Controlling on-surface polymerization by hierarchical and substrate-directed growth. Nat. Chem. 4, 215–220 (2012).
McCarty, G. S. & Weiss, P. S. Formation and manipulation of protopolymer chains. J. Am. Chem. Soc. 126, 16772–16776 (2004).
Blake, M. M. et al. Identifying reactive intermediates in the Ullmann coupling reaction by scanning tunneling microscopy and spectroscopy. J. Phys. Chem. A 113, 13167–13172 (2009).
Lipton-Duffin, J. A., Ivasenko, O., Perepichka, D. F. & Rosei, F. Synthesis of polyphenylene molecular wires by surface-confined polymerization. Small 5, 592–597 (2009).
Di Giovannantonio, M. et al. Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization. ACS Nano 7, 8190–8198 (2013).
Lipton-Duffin, J. A. et al. Step-by-step growth of epitaxially aligned polythiophene by surface-confined reaction. Proc. Natl Acad. Sci. USA 107, 11200–11204 (2010).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Bieri, M. et al. Surface-supported 2D heterotriangulene polymers. Chem. Commun. 47, 10239–10241 (2011).
Bieri, M. et al. Two-dimensional polymer formation on surfaces: insight into the roles of precursor mobility and reactivity. J. Am. Chem. Soc. 132, 16669–16676 (2010).
Cardenas, L. et al. Synthesis and electronic structure of a two dimensional π-conjugated polythiophene. Chem. Sci. 4, 3263–3268 (2013).
Eichhorn, J. et al. On-surface Ullmann coupling: the influence of kinetic reaction parameters on the morphology and quality of covalent networks. ACS Nano 8, 7880–7889 (2014).
Moreno, C. et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Di Giovannantonio, M. & Contini, G. Reversibility and intermediate steps as key tools for the growth of extended ordered polymers via on-surface synthesis. J. Phys. Condens. Matter 30, 093001 (2018).
Zhong, Y. et al. Wafer-scale synthesis of monolayer two-dimensional porphyrin polymers for hybrid superlattices. Science 366, 1379–1384 (2019).
Agarwala, P. & Kabra, D. A review on triphenylamine (TPA) based organic hole transport materials (HTMs) for dye sensitized solar cells (DSSCs) and perovskite solar cells (PSCs): evolution and molecular engineering. J. Mater. Chem. A 5, 1348–1373 (2017).
Cai, L. et al. Organic sensitizers with bridged triphenylamine donor units for efficient dye-sensitized solar cells. Adv. Energy Mater. 3, 200–205 (2013).
Ohta, T. et al. Interlayer interaction and electronic screening in multilayer graphene investigated with angle-resolved photoemission spectroscopy. Phys. Rev. Lett. 98, 206802 (2007).
Kang, M. et al. Dirac fermions and flat bands in the ideal kagome metal FeSn. Nat. Mater. 19, 163–169 (2020).
Liu, Y., Bian, G., Miller, T. & Chiang, T. C. Visualizing electronic chirality and Berry phases in graphene systems using photoemission with circularly polarized light. Phys. Rev. Lett. 107, 166803 (2011).
Tesch, J. et al. Structural and electronic properties of graphene nanoflakes on Au(111) and Ag(111). Sci. Rep. 6, 23439 (2016).
Paniago, R., Matzdorf, R., Meister, G. & Goldmann, A. Temperature dependence of Shockley-type surface energy bands on Cu(111), Ag(111) and Au(111). Surf. Sci. 336, 113–122 (1995).
Steiner, C. et al. Hierarchical on-surface synthesis and electronic structure of carbonyl-functionalized one- and two-dimensional covalent nanoarchitectures. Nat. Commun. 8, 14765 (2017).
Galeotti, G. et al. Surface-mediated assembly, polymerization and degradation of thiophene-based monomers. Chem. Sci. 10, 5167–5175 (2019).
Blunt, M. O., Russell, J. C., Champness, N. R. & Beton, P. H. Templating molecular adsorption using a covalent organic framework. Chem. Commun. 46, 7157–7159 (2010).
Di Giovannantonio, M. et al. Mechanistic picture and kinetic analysis of surface-confined Ullmann polymerization. J. Am. Chem. Soc. 138, 16696–16702 (2016).
De Marchi, F. et al. Temperature-induced molecular reorganization on Au(111) driven by oligomeric defects. Nanoscale 11, 19468–19476 (2019).
Schlögl, S., Heckl, W. M. & Lackinger, M. On-surface radical addition of triply iodinated monomers on Au(111)—the influence of monomer size and thermal post-processing. Surf. Sci. 606, 999–1004 (2012).
Suzuki, S. et al. Trinitroxide-trioxytriphenylamine: spin-state conversion from triradical doublet to diradical cation triplet by oxidative modulation of a π-conjugated system. Angew. Chem. Int. Ed. 51, 3193–3197 (2012).
Fang, Z. et al. Bridged triphenylamine-based dendrimers: tuning enhanced two-photon absorption performance with locked molecular planarity. Org. Lett. 11, 1–4 (2009).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Frisch, M. J. et al. Gaussian 16 revision b.01 (Gaussian, Inc., 2016).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Heyd, J., Peralta, J. E., Scuseria, G. E. & Martin, R. L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 123, 174101 (2005).
Acknowledgements
This work was partially supported by the project Grande Rilevanza Italy-Quebec of the Italian Ministero degli Affari Esteri e della Cooperazione Internazionale (MAECI), Direzione Generale per la Promozione del Sistema Paese. M.C.G., D.F.P. and F.R. acknowledge funding from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQNRT) through a Team Grant. The work at McGill was supported by the US Army Research Office (grant W911NF-17-1-0126). F.R. is grateful to the Canada Research Program for funding and partial salary support. The authors acknowledge beamtime access and support from the Elettra light source in Italy. We thank N. Preetha Genesh for help with ambient STM imaging. G.G., F.F. and G.C. thank N. Zema for laboratory support and useful discussions. Computations were performed mostly on the Niagara supercomputer at the SciNet HPC Consortium, funded by the Canada Foundation for Innovation under Compute Canada, the Government of Ontario, Ontario Research Fund – Research Excellence and the University of Toronto, and in part on the Graham and Cedar clusters of Compute Canada. The simulations were also enabled by the facilities of the Shared Hierarchical Academic Research Computing Network as well as WestGrid. A.K.K., P.M.S. and P.M. acknowledge the project EUROFEL-ROADMAP ESFRI.
Author information
Authors and Affiliations
Contributions
G.G., F.D.M. and G.C. wrote the paper; G.G. and F.D.M performed laboratory XPS and STM experiments and analysed the data; M.C.G. and D.D. participated in STM and laboratory XPS data acquisition and analysis; E.H., M.R.R. and Y.C. synthesized the molecules; O.M., E.H. and M.E. performed the DFT calculations; L.V.B. performed the statistical analysis of the STM images; G.G., G.C., P.M.S., P.M., A.K.K., F.F. and L.F. performed the synchrotron measurements, and G.G., G.C., P.M.S., P.M. and L.F. discussed and analysed the data; G.G., D.D., F.F., R.L., M.C.G. and G.C. performed and analysed the LEED experiments; G.C., M.C.G., F.R. and D.F.P. conceived the experiments and supervised the work; all authors participated in editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, discussion and synthesis procedures.
Rights and permissions
About this article
Cite this article
Galeotti, G., De Marchi, F., Hamzehpoor, E. et al. Synthesis of mesoscale ordered two-dimensional π-conjugated polymers with semiconducting properties. Nat. Mater. 19, 874–880 (2020). https://doi.org/10.1038/s41563-020-0682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0682-z
This article is cited by
-
Universal inter-molecular radical transfer reactions on metal surfaces
Nature Communications (2024)
-
Topology selectivity of a conformationally flexible precursor through selenium doping
Nature Communications (2024)
-
Deceptive orbital confinement at edges and pores of carbon-based 1D and 2D nanoarchitectures
Nature Communications (2024)
-
Artificial kagome lattices of Shockley surface states patterned by halogen hydrogen-bonded organic frameworks
Nature Communications (2024)
-
Prediction of highly stable 2D carbon allotropes based on azulenoid kekulene
Nature Communications (2024)