Abstract
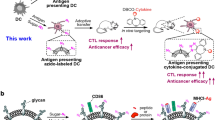
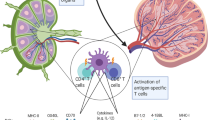
Targeted immunomodulation of dendritic cells (DCs) in vivo will enable manipulation of T-cell priming and amplification of anticancer immune responses, but a general strategy has been lacking. Here we show that DCs concentrated by a biomaterial can be metabolically labelled with azido groups in situ, which allows for their subsequent tracking and targeted modulation over time. Azido-labelled DCs were detected in lymph nodes for weeks, and could covalently capture dibenzocyclooctyne (DBCO)-bearing antigens and adjuvants via efficient Click chemistry for improved antigen-specific CD8+ T-cell responses and antitumour efficacy. We also show that azido labelling of DCs allowed for in vitro and in vivo conjugation of DBCO-modified cytokines, including DBCO–IL-15/IL-15Rα, to improve priming of antigen-specific CD8+ T cells. This DC labelling and targeted modulation technology provides an unprecedented strategy for manipulating DCs and regulating DC–T-cell interactions in vivo.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data supporting the results are provided with the manuscript. Raw datasets are available at https://dataverse.harvard.edu/privateurl.xhtml?token=4d292632-f627-4a56-8e01-60e36d0883f5.
References
Laughlin, S. T. & Bertozzi, C. R. Metabolic labeling of glycans with azido sugars and subsequent glycan-profiling and visualization via staudinger ligation. Nat. Protoc. 2, 2930 (2007).
Laughlin, S. T. et al. Metabolic labeling of glycans with azido sugars for visualization and glycoproteomics. Methods Enzymol. 415, 230–250 (2006).
Chang, P. V. et al. Copper-free click chemistry in living animals. Proc. Natl Acad. Sci. USA 107, 1821–1826 (2010).
Baskin, J. M. et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl Acad. Sci. USA 104, 16793–16797 (2007).
Wang, H. et al. Targeted ultrasound-assisted cancer-selective chemical labeling and subsequent cancer imaging using click chemistry. Angew. Chem. Int. Ed. 55, 5452–5456 (2016).
Wang, H. et al. In vivo cancer targeting via glycopolyester nanoparticle mediated metabolic cell labeling followed by click reaction. Biomaterials 218, 119305 (2019).
Lee, S. et al. Chemical tumor-targeting of nanoparticles based on metabolic glycoengineering and click chemistry. ACS Nano 8, 2048–2063 (2014).
Du, L., Qin, H., Ma, T., Zhang, T. & Xing, D. In vivo imaging-guided photothermal/photoacoustic synergistic therapy with bioorthogonal metabolic glycoengineering-activated tumor targeting nanoparticles. ACS Nano 11, 8930–8943 (2017).
Li, X., Xu, X., Rao, X., Tian, Y. & Yi, W. Chemical remodeling cell surface glycans for immunotargeting of tumor cells. Carbohydr. Res. 452, 25–34 (2017).
Le Gall, C. M., Weiden, J., Eggermont, L. J. & Figdor, C. G. Dendritic cells in cancer immunotherapy. Nat. Mater. 17, 474–475 (2018).
Sallusto, F. et al. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 29, 1617–1625 (1999).
Liu, Y.-J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Merad, M., Sathe, P., Helft, J., Miller, J. & Mortha, A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu. Rev. Immunol. 31, 563–604 (2013).
De Vries, I. J. M. et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat. Biotechnol. 23, 1407–1413 (2005).
Rhee, I., Zhong, M.-C., Reizis, B., Cheong, C. & Veillette, A. Control of dendritic cell migration, T cell-dependent immunity, and autoimmunity by protein tyrosine phosphatase PTPN12 expressed in dendritic cells. Mol. Cell Biol. 34, 888–899 (2014).
Ahrens, E. T. & Bulte, J. W. Tracking immune cells in vivo using magnetic resonance imaging. Nat. Rev. Immunol. 13, 755–763 (2013).
Bertozzi, C. R. & Kiessling, L. Chemical glycobiology. Science 291, 2357–2364 (2001).
Luchansky, S. J. et al. in Methods of Enzymology Vol. 362 (eds Lee Yuan, C. & Lee Reiko, T.) 249–272 (Academic Press, 2003).
Prescher, J. A. & Bertozzi, C. R. Chemistry in living systems. Nat. Chem. Biol. 1, 13–21 (2005).
Kang, S.-W. et al. Cell labeling and tracking method without distorted signals by phagocytosis of macrophages. Theranostics 4, 420–431 (2014).
Verbeke, C. S. & Mooney, D. J. Injectable, pore-forming hydrogels for in vivo enrichment of immature dendritic cells. Adv. Healthc. Mater. 4, 2677–2687 (2015).
Verbeke, C. S. et al. Multicomponent injectable hydrogels for antigen-specific tolerogenic immune modulation. Adv. Healthc. Mater. 6, https://doi.org/10.1002/adhm.201600773 (2017).
Wang, H. et al. In vivo targeting of metabolically labeled cancers with ultra-small silica nanoconjugates. Theranostics 6, 1467–1476 (2016).
Kearney, C. J. et al. Switchable release of entrapped nanoparticles from alginate hydrogels. Adv. Healthc. Mater. 4, 1634–1639 (2015).
Kamath, A. T., Henri, S., Battye, F., Tough, D. F. & Shortman, K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100, 1734–1741 (2002).
Randolph, G. J., Angeli, V. & Swartz, M. A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Wang, H. et al. Selective in vivo metabolic cell-labeling-mediated cancer targeting. Nat. Chem. Biol. 13, 415–424 (2017).
Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 4, 11–22 (2004).
Motzer, R. J. et al. Effect of cytokine therapy on survival for patients with advanced renal cell carcinoma. J. Clin. Oncol. 18, 1928–1935 (2000).
Waldmann, T. A. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 6, 595–601 (2006).
Sato, N., Patel, H. J., Waldmann, T. A. & Tagaya, Y. The IL-15/IL-15Rα on cell surfaces enables sustained IL-15 activity and contributes to the long survival of CD8 memory T cells. Proc. Natl Acad. Sci. USA 104, 588–593 (2007).
Stoklasek, T. A., Schluns, K. S. & Lefrançois, L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177, 6072–6080 (2006).
Ferlazzo, G. et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc. Natl Acad. Sci. USA 101, 16606–16611 (2004).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Epardaud, M. et al. Interleukin-15/Interleukin-15Rα complexes promote destruction of established tumors by reviving tumor-resident CD8+ T Cells. Cancer Res. 68, 2972–2983 (2008).
Bonifaz, L. C. et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J. Exp. Med. 199, 815–824 (2004).
Tacken, P. J., de Vries, I. J. M., Torensma, R. & Figdor, C. G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat. Rev. Immunol. 7, 790–802 (2007).
Liu, H. et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 507, 519–522 (2014).
Reddy, S. T., Rehor, A., Schmoekel, H. G., Hubbell, J. A. & Swartz, M. A. In vivo targeting of dendritic cells in lymph nodes with poly(propylene sulfide) nanoparticles. J. Control. Release 112, 26–34 (2006).
Tomura, M. et al. Monitoring cellular movement in vivo with photoconvertible fluorescence protein ‘Kaede’ transgenic mice. Proc. Natl Acad. Sci. USA 105, 10871–10876 (2008).
Tomura, M. et al. Tracking and quantification of dendritic cell migration and antigen trafficking between the skin and lymph nodes. Sci. Rep. 4, 6030 (2014).
Alvarez, D., Vollmann, E. H. & von Andrian, U. H. Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342 (2008).
Ichihashi, M. et al. UV-induced skin damage. Toxicology 189, 21–39 (2003).
Chang, P. V., Dube, D. H., Sletten, E. M. & Bertozzi, C. R. A strategy for the selective imaging of glycans using caged metabolic precursors. J. Am. Chem. Soc. 132, 9516–9518 (2010).
Hanson, M. C. et al. Nanoparticulate STING agonists are potent lymph node–targeted vaccine adjuvants. J. Clin. Invest. 125, 2532–2546 (2015).
Mueller, S. N., Tian, S. & DeSimone, J. M. Rapid and persistent delivery of antigen by lymph node targeting PRINT nanoparticle vaccine carrier to promote humoral immunity. Mol. Pharm. 12, 1356–1365 (2015).
Wang, Q., Cao, W., Yang, Z.-G. & Zhao, G.-F. DC targeting DNA vaccines induce protective and therapeutic antitumor immunity in mice. Int. J. Clin. Exp. Med. 8, 17565–17577 (2015).
Cruz, L. J. et al. Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8+ T cell response: a comparative study. J. Control. Release 192, 209–218 (2014).
Cheong, C. et al. Improved cellular and humoral immune responses in vivo following targeting of HIV Gag to dendritic cells within human anti-human DEC205 monoclonal antibody. Blood 116, 3828–3838 (2010).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Wang, H. & Mooney, D. J. Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater. 17, 761–772 (2018).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
Hodi, F. S. Dendritic cell activating scaffold in melanoma. ClinicalTrials.org https://clinicaltrials.gov/show/nct01753089 (2017).
Acknowledgements
We acknowledge funding from the National Institutes of Health (grant nos. U01 CA214369 and R01 CA223255). H.W. gratefully acknowledges funding support from the Wyss Technology Development Fellowship. M.C.S. and C.M.T. acknowledge funding support from the Graduate Research Fellowship Program from the National Science Foundation. D.K.Y.Z. acknowledges support from the Canadian Institutes of Health Research. We thank A. J. Najibi at Harvard University for discussions.
Author information
Authors and Affiliations
Contributions
H.W. and D.J.M. conceived the study, designed the experiments and wrote the manuscript. H.W., M.C.S., D.K.Y.Z., A.N.C., A.W.L., M.O.D., C.M.T. and S.K. carried out the experiments. K.W.W. designed IL-15/IL-15R𝛂 and contributed to the design of IL-15/IL-15R𝛂 study.
Corresponding author
Ethics declarations
Competing interests
D.J.M. conducts research sponsored by Novartis, Merck, Decibel and Amgen. D.J.M. consults for Agnovos and the Samyang Corporation. D.J.M. holds equity in Immulus. H.W. and D.J.M. are inventors of a patent application on the labelling technology.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16.
Rights and permissions
About this article
Cite this article
Wang, H., Sobral, M.C., Zhang, D.K.Y. et al. Metabolic labeling and targeted modulation of dendritic cells. Nat. Mater. 19, 1244–1252 (2020). https://doi.org/10.1038/s41563-020-0680-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0680-1
This article is cited by
-
In situ PEGylation of CAR T cells alleviates cytokine release syndrome and neurotoxicity
Nature Materials (2023)
-
Metabolic glycan labeling immobilizes dendritic cell membrane and enhances antitumor efficacy of dendritic cell vaccine
Nature Communications (2023)
-
Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines
Nature Communications (2023)
-
Capturing nascent extracellular vesicles by metabolic glycan labeling-assisted microfluidics
Nature Communications (2023)
-
Optimizing the manufacturing and antitumour response of CAR T therapy
Nature Reviews Bioengineering (2023)