Abstract
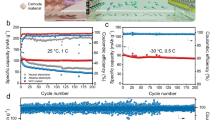
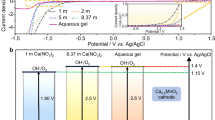
Developing low-cost and eco-friendly aqueous electrolytes with a wide voltage window is critical to achieve safe, high-energy and sustainable Li-ion batteries. Emerging approaches using highly concentrated salts (21–55 m (mol kg–1)) create artificial solid–electrode interfaces and improve water stability; however, these approaches raise concerns about cost and toxicity. Molecular crowding is a common phenomenon in living cells where water activity is substantially suppressed by molecular crowding agents through altering the hydrogen-bonding structure. Here we demonstrate a ‘molecular crowding’ electrolyte using the water-miscible polymer poly(ethylene glycol) as the crowding agent to decrease water activity, thereby achieving a wide electrolyte operation window (3.2 V) with low salt concentration (2 m). Aqueous Li4Ti5O12/LiMn2O4 full cells with stable specific energies between 75 and 110 W h kg−1 were demonstrated over 300 cycles. Online electrochemical mass spectroscopy revealed that common side reactions in aqueous Li-ion batteries (hydrogen/oxygen evolution reactions) are virtually eliminated. This work provides a path for designing high-voltage aqueous electrolytes for low-cost and sustainable energy storage.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Armand, M. & Tarascon, J. M. Building better batteries. Nature 451, 652–657 (2008).
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Grey, C. P. & Tarascon, J. M. Sustainability and in situ monitoring in battery development. Nat. Mater. 16, 45–56 (2017).
Li, W., Dahn, J. R. & Wainwright, D. S. Rechargeable lithium batteries with aqueous electrolytes. Science 264, 1115–1118 (1994).
Kim, H. et al. Aqueous rechargeable Li and Na ion batteries. Chem. Rev. 114, 11788–11827 (2014).
Wang, Y., Yi, J. & Xia, Y. Recent progress in aqueous lithium-ion batteries. Adv. Energy Mater. 2, 830–840 (2012).
Suo, L. et al. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Suo, L. et al. Advanced high-voltage aqueous lithium-ion battery enabled by “water-in-bisalt” electrolyte. Angew. Chem. Int. Ed. 55, 7136–7141 (2016).
Yamada, Y. et al. Hydrate-melt electrolytes for high-energy-density aqueous batteries. Nat. Energy 1, 16129 (2016).
Ko, S. et al. Lithium-salt monohydrate melt: a stable electrolyte for aqueous lithium-ion batteries. Electrochem. Commun. 104, 106488 (2019).
Wang, F. et al. Stabilizing high voltage LiCoO2 cathode in aqueous electrolyte with interphase-forming additive. Energy Environ. Sci. 9, 3666–3673 (2016).
Wang, F. et al. Spinel LiNi0.5Mn1.5O4 cathode for high-energy aqueous lithium-ion batteries. Adv. Energy Mater. 7, 1600922 (2017).
Wang, F. et al. Hybrid aqueous/non-aqueous electrolyte for safe and high-energy Li-ion batteries. Joule 2, 927–937 (2018).
Yang, C. et al. 4.0 V aqueous Li-ion batteries. Joule 1, 122–132 (2017).
Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. Nature 569, 245–250 (2019).
Yamada, Y., Wang, J., Ko, S., Watanabe, E. & Yamada, A. Advances and issues in developing salt-concentrated battery electrolytes. Nat. Energy 4, 269–280 (2019).
Lukatskaya, M. R. et al. Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 11, 2876–2883 (2018).
He, X. et al. Fluorine-free water-in-ionomer electrolytes for sustainable lithium-ion batteries. Nat. Commun. 9, 5320 (2018).
Fulton, A. B. How crowded is the cytoplasm? Cell 30, 345–347 (1982).
Kuznetsova, I. M., Turoverov, K. K. & Uversky, V. N. What macromolecular crowding can do to a protein. Int. J. Mol. Sci. 15, 23090–23140 (2014).
Nakano, S. I., Miyoshi, D. & Sugimoto, N. Effects of molecular crowding on the structures, interactions, and functions of nucleic acids. Chem. Rev. 114, 2733–2758 (2014).
Kilburn, D., Roh, J. H., Guo, L., Briber, R. M. & Woodson, S. A. Molecular crowding stabilizes folded RNA structure by the excluded volume effect. J. Am. Chem. Soc. 132, 8690–8696 (2010).
Gao, M. et al. RNA hairpin folding in the crowded cell. Angew. Chem. Int. Ed. 55, 3224–3228 (2016).
Ferreira, L. A., Uversky, V. N. & Zaslavsky, B. Y. Role of solvent properties of water in crowding effects induced by macromolecular agents and osmolytes. Mol. Biosyst. 13, 2551–2563 (2017).
Ensing, B. et al. On the origin of the extremely different solubilities of polyethers in water. Nat. Commun. 10, 2893 (2019).
Verma, P. K., Kundu, A., Ha, J. H. & Cho, M. Water dynamics in cytoplasm-like crowded environment correlates with the conformational transition of the macromolecular crowder. J. Am. Chem. Soc. 138, 16081–16088 (2016).
Xue, Z., He, D. & Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 3, 19218–19253 (2015).
Whitehead, A. H. & Schreiber, M. Current collectors for positive electrodes of lithium-based batteries. J. Electrochem. Soc. 152, A2105–A2113 (2005).
Kühnel, R. S. et al. “Water-in-salt” electrolytes enable the use of cost-effective aluminum current collectors for aqueous high-voltage batteries. Chem. Commun. 52, 10435–10438 (2016).
Jora, M. Z., Cardoso, M. V. & Sabadini, E. Dynamical aspects of water-poly(ethylene glycol) solutions studied by 1H NMR. J. Mol. Liq. 222, 94–100 (2016).
Pavelec, J., DiGuiseppi, D., Zavlavsky, B. Y., Uversky, V. N. & Schweitzer-Stenner, R. Perturbation of water structure by water-polymer interactions probed by FTIR and polarized Raman spectroscopy. J. Mol. Liq. 275, 463–473 (2019).
Huheey, J. E. Electronegativity, acids, and bases. IV. Concerning the inductive effect of alkyl groups. J. Org. Chem. 36, 204–205 (1971).
Eisenberg, D. & Kauzmann, W. The Structure and Properties of Water Ch. 4 (Oxford Univ. Press, 2005).
Liang, J.-Y. et al. Mitigating interfacial potential drop of cathode–solid electrolyte via ionic conductor layer to enhance interface dynamics for solid batteries. J. Am. Chem. Soc. 140, 6767–6770 (2018).
Liang, Z. & Lu, Y.-C. Critical role of redox mediator in suppressing charging instabilities of lithium–oxygen batteries. J. Am. Chem. Soc. 138, 7574–7583 (2016).
Liang, Z., Zhou, Y. & Lu, Y.-C. Dynamic oxygen shield eliminates cathode degradation in lithium–oxygen batteries. Energy Environ. Sci. 11, 3500–3510 (2018).
Dubouis, N. et al. The role of the hydrogen evolution reaction in the solid–electrolyte interphase formation mechanism for “water-in-salt” electrolytes. Energy Environ. Sci. 11, 3491–3499 (2018).
Suen, N.-T. et al. Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem. Soc. Rev. 46, 337–365 (2017).
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Fan, L., Wei, S., Li, S., Li, Q. & Lu, Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 8, 1702657 (2018).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1997).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blandamer, M. J., Fox, M. F., Powell, E. & Stafford, J. W. A viscometric study of poly(ethylene oxide) in t-butyl alcohol/water mixtures. Macromol. Chem. Phys. 124, 222–231 (1969).
Acknowledgements
The work described in this paper was fully supported by a grant from the Research Grant Council of the Hong Kong Special Administrative Region, China (project no. CUHK14307318). We thank G. T. Cong for assisting with the DFT calculations, Y. C. Zhou for assisting with the electrode fabrications and W. W. Wang for recording videos.
Author information
Authors and Affiliations
Contributions
J.X. and Y.-C.L. conceived the project, analysed the data and wrote the manuscript. J.X. performed the DFT calculation and conducted the experiments with contributions from Z.L. (OEMS measurement).
Corresponding author
Ethics declarations
Competing interests
J.X. and Y.-C.L. are inventors with a patent application (US application no. 16/798,136) on the molecular crowding electrolytes described herein. Z.L. declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Fabrication of 2 m LiTFSI-xPEG-(1-x)H2O electrolytes.
Stoichiometric amounts of water, PEG 400 and LiTFSI were used to prepare 2 m LiTFSI-xPEG-(1-x)H2O (x=94% is shown as an example). All the solutions were fabricated in an argon-filled glove box at room temperature after bubbling water and PEG 400 with argon for 10 minutes to prevent oxygen contamination on electrodes.
Extended Data Fig. 2 Ionic conductivity of the constructed “molecular crowding” electrolytes.
The ionic conductivity of 2 m LiTFSI-xPEG-(1-x)H2O was measured at 25 oC, x= 71, 80, 90, 94%,100%.
Extended Data Fig. 3 Flammability testing.
a, An ignited cotton swab was immersed in the commercial 1 M LiPF6 in EC/DEC(vol%=1/1) and 2 m LiTFSI-94%PEG-6%H2O. 1 M LiPF6 in EC/DEC(vol%=1/1) electrolyte was ignited immediately, while the fire of cotton swab was extinguished in 2 m LiTFSI-94%PEG-6%H2O. b, The snapshot of Supplementary Video 3 compares the flammability of glass fibre soaked with 2 m LiTFSI-94%PEG-6%H2O, 2 m LiTFSI-PEG and the film of 2 m LiTFSI-PEO after treated with propane flame. The full process is shown in Supplementary Video 3.
Extended Data Fig. 4 1H-NMR spectra.
1H-NMR spectra of 2 m LiTFSI-xPEG-(1-x)H2O, x= 0%, 71%, 80%, 90%, 94%. The 1H shift of H2O decreases upon PEG addition.
Extended Data Fig. 5 Snapshots of 2 m LiTFSI-94%PEG-6%H2O and 2 m LiTFSI-H2O electrolyte during MD simulations.
a, The snapshot of 2 m LiTFSI-94%PEG-6%H2O electrolyte during 20 ps-120 ps of the simulation. b, The snapshot of 2 m LiTFSI-H2O electrolyte during 4 ps-10 ps of the simulation.
Extended Data Fig. 6 Cell design of online electrochemical mass spectrometry.
The cell design is adopted from our previous work35,36. To avoid the fast evaporation of water in the electrolyte with continuous gas flowing, the Ar gas was directed into a blank cell(right one) which consists of only electrolyte (without electrodes) for gas washing before flowing into the electrochemical cell(left one).
Extended Data Fig. 7 Comparison of electrochemical stability window obtained from different working electrodes.
a, Electrochemical stability windows of hydrate melt 19.4 m LiTFSI-8.3 m LiBETI determined by liner sweep voltammetry tests on AB coated Al foil and bare Al/Ti current collector (Al for cathodic scanning and Ti for anodic scanning, which were used in ref. 9). b, Electrochemical stability windows of 32 m KAc-8 m LiAc determined by liner sweep voltammetry tests on AB coated Al foil and bare Ti current collector (adopted in ref. 17).
Extended Data Fig. 8 Nyquist plots of electrochemical impedance spectroscopy(EIS) for the LTO/LMO full cell with 2 m LiTFSI-94%PEG-6%H2O and 2 m LiTFSI-PEO at 25 oC.
The Nyquist plots of electrochemical impedance spectroscopy(EIS) for the LTO/LMO full cell with 2 m LiTFSI-94%PEG-6%H2O and 2 m LiTFSI-PEO at 25 oC shows that the interfacial resistance of 2 m LiTFSI-94%PEG-6%H2O is much smaller than polymer electrolyte 2 m LiTFSI-PEO.
Extended Data Fig. 9 Voltage profile of L-LTO/LMO and gel pre-coated Li/LMO at 1 C in 2 m LiTFSI-94%PEG-6%H2O electrolyte during the 1st cycle.
The voltage profile of L-LTO/LMO and gel pre-coated Li/LMO at 1 C in 2 m LiTFSI-94%PEG-6%H2O electrolyte during the 1st cycle shows the potential application of our electrolyte for 4.0 V aqueous battery.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, Tables 1–3, Discussion I, captions for Videos 1–3 and refs. 1–15.
Supplementary Video 1
Flammability testing of the 2 m LiTFSI–94%PEG–6%H2O electrolyte. The fire of an ignited cotton swab was extinguished after being immersed in the 2 m LiTFSI–94%PEG–6%H2O electrolyte, suggesting that the electrolyte is non-flammable and safe.
Supplementary Video 2
Flammability testing of commercial LiPF6 in EC/DEC electrolyte. The commercial LiPF6 in the EC/DEC electrolyte started to burn after an ignited cotton swab was immersed in the electrolyte, indicating that the electrolyte is flammable.
Supplementary Video 3
The comparison of the flammability of 2 m LiTFSI–94%PEG–6%H2O, 2 m LiTFSI–PEG and 2 m LiTFSI–PEO. The 2 m LiTFSI–94%PEG–6%H2O-soaked glass fibre was not ignited, while those soaked in 2 m LiTFSI–PEG and polymer electrolyte 2 m LiTFSI–PEO (EO/Li+, 11:1) were both ignited, indicating that the 2 m LiTFSI–94%PEG–6%H2O exhibits superior safety compared with the 2 m LiTFSI–PEG and solid PEO polymer electrolyte.
Rights and permissions
About this article
Cite this article
Xie, J., Liang, Z. & Lu, YC. Molecular crowding electrolytes for high-voltage aqueous batteries. Nat. Mater. 19, 1006–1011 (2020). https://doi.org/10.1038/s41563-020-0667-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0667-y
This article is cited by
-
Water-in-polymer electrolyte with a wide electrochemical window and recyclability
Nature Sustainability (2024)
-
Asymmetric Electrolytes Design for Aqueous Multivalent Metal Ion Batteries
Nano-Micro Letters (2024)
-
Trend of Developing Aqueous Liquid and Gel Electrolytes for Sustainable, Safe, and High-Performance Li-Ion Batteries
Nano-Micro Letters (2024)
-
Design strategies for rechargeable aqueous metal-ion batteries
Science China Chemistry (2024)
-
An Electrochemical Perspective of Aqueous Zinc Metal Anode
Nano-Micro Letters (2024)