Abstract
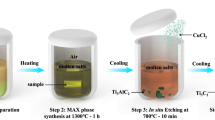
Two-dimensional carbides and nitrides of transition metals, known as MXenes, are a fast-growing family of materials that have attracted attention as energy storage materials. MXenes are mainly prepared from Al-containing MAX phases (where A = Al) by Al dissolution in F-containing solution; most other MAX phases have not been explored. Here a redox-controlled A-site etching of MAX phases in Lewis acidic melts is proposed and validated by the synthesis of various MXenes from unconventional MAX-phase precursors with A elements Si, Zn and Ga. A negative electrode of Ti3C2 MXene material obtained through this molten salt synthesis method delivers a Li+ storage capacity of up to 738 C g−1 (205 mAh g−1) with high charge–discharge rate and a pseudocapacitive-like electrochemical signature in 1 M LiPF6 carbonate-based electrolyte. MXenes prepared via this molten salt synthesis route may prove suitable for use as high-rate negative-electrode materials for electrochemical energy storage applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
18 January 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41563-021-00925-4
References
Naguib, M. et al. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 23, 4248–4253 (2011).
Lukatskaya, M. R. et al. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 341, 1502–1505 (2013).
Anasori, B., Lukatskaya, M. R. & Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2, 16098 (2017).
Ghidiu, M., Lukatskaya, M. R., Zhao, M.-Q., Gogotsi, Y. & Barsoum, M. W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 516, 78–81 (2014).
Feng, A. et al. Fabrication and thermal stability of NH4HF2-etched Ti3C2 MXene. Ceram. Int. 43, 6322–6328 (2017).
Li, M. et al. Element replacement approach by reaction with Lewis acidic molten salts to synthesize nanolaminated MAX phases and MXenes. J. Am. Chem. Soc. 141, 4730–4737 (2019).
Naoi, K. et al. Ultrafast charge-discharge characteristics of a nanosized core-shell structured LiFePO4 material for hybrid supercapacitor applications. Energy Environ. Sci. 9, 2143–2151 (2016).
Lukatskaya, M. R., Dunn, B. & Gogotsi, Y. Multidimensional materials and device architectures for future hybrid energy storage. Nat. Commun. 7, 12647 (2016).
Alhabeb, M. et al. Selective etching of silicon from Ti3SiC2 (MAX) to obtain 2D titanium carbide (MXene). Angew. Chem. Int. Ed. 130, 5542–5546 (2018).
Yang, S. et al. Ultrafast delamination of graphite into high-quality graphene using alternating currents. Angew. Chem. Int. Ed. 56, 6669–6675 (2017).
Kisi, E., Crossley, J., Myhra, S. & Barsoum, M. Structure and crystal chemistry of Ti3SiC2. J. Phys. Chem. Solids 59, 1437–1443 (1998).
Halim, J. et al. X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes). Appl. Surf. Sci. 362, 406–417 (2016).
Çakır, O. Review of etchants for copper and its alloys in wet etching processes. Key Eng. Mater. 364-366, 460–465 (2008).
Shpigel, N. et al. Direct assessment of nanoconfined water in 2D Ti3C2 electrode interspaces by a surface acoustic technique. J. Am. Chem. Soc. 140, 8910–8917 (2018).
Lu, J. et al. Tin+1Cn MXenes with fully saturated and thermally stable Cl terminations. Nanoscale Adv. 9, 3680–3685 (2019).
Khazaei, M. et al. Insights into exfoliation possibility of MAX phases to MXenes. Phys. Chem. Chem. Phys. 20, 8579–8592 (2018).
Wang, X. et al. Influences from solvents on charge storage in titanium carbide MXenes. Nat. Energy 4, 241–248 (2019).
Wang, X. et al. Pseudocapacitance of MXene nanosheets for high-power sodium-ion hybrid capacitors. Nat. Commun. 6, 6544 (2015).
Come, J. et al. A non-aqueous asymmetric cell with a Ti2C-based two-dimensional negative electrode. J. Electrochem. Soc. 159, A1368–A1373 (2012).
Ren, C. E. et al. Porous two-dimensional transition metal carbide (MXene) flakes for high-performance Li-Ion storage. ChemElectroChem 3, 689–693 (2016).
Cheng, R. et al. Understanding the lithium storage mechanism of Ti3C2Tx MXene. J. Phys. Chem. C 123, 1099–1109 (2019).
Xie, Y. et al. Role of surface structure on Li-ion energy storage capacity of two-dimensional transition-metal carbides. J. Am. Chem.Soc. 136, 6385–6394 (2014).
Kajiyama, S. et al. Enhanced Li‐ion accessibility in MXene titanium carbide by steric chloride termination. Adv. Energy Mater. 7, 1601873 (2017).
Zhao, J. et al. One-step synthesis of few-layer niobium carbide MXene as a promising anode material for high-rate lithium ion batteries. Dalton Trans. 48, 14433–14439 (2019).
Pang, J. et al. Applications of 2D MXenes in energy conversion and storage systems. Chem. Soc. Rev. 48, 72–133 (2019).
Xiong, D., Li, X., Bai, Z. & Lu, S. Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 14, 1703419 (2018).
Lukatskaya, M. R. et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2, 17105 (2017).
Birkl, C. R., Roberts, M. R., McTurk, E., Bruce, P. G. & Howey, D. A. Degradation diagnostics for lithium ion cells. J. Power Sources 341, 373–386 (2017).
Lukatskaya, M. R. et al. Probing the mechanism of high capacitance in 2D titanium carbide using in situ X-ray absorption spectroscopy. Adv. Energy Mater. 5, 1500589 (2015).
Augustyn, V. et al. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 12, 518–522 (2013).
Kim, H.-S. et al. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3-x. Nat. Mater. 16, 454–460 (2017).
Diard, J. P., Gorrec, B. L. & Montella, C. Linear diffusion impedance. General expression and applications. J. Electroanal. Chem. 471, 126–131 (1999).
Morcrette, M. et al. In situ X-ray diffraction techniques as a powerful tool to study battery electrode materials. Electrochim. Acta 47, 3137–3149 (2002).
Acknowledgements
This study was supported financially by the National Natural Science Foundation of China (grant nos. 21671195, 91426304, 51902320 and 51902319) and by the China Postdoctoral Science Foundation (grant no. 2018M642498). H.S. was supported by a grant from the China Scolarship Council. P.S., P.L.T. and H.S. thank the Agence Nationale de la Recherche (Labex STORE-EX) for financial support. Q.H. thanks the International Partnership Program of Chinese Academy of Sciences (grant no. 174433KYSB20190019), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang, and the Ningbo top-talent team program for financial support. Z.L. is supported by the Fundamental Research Funds for the Central Universities (grant no. YJ201886). We acknowledge the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (faculty grant SFO‐Mat‐LiU no. 2009 00971). The Knut and Alice Wallenberg Foundation is acknowledged for support of the electron microscopy laboratory in Linköping, a Fellowship grant (P.E) and a scholar grant (L.H., 2016-0358).
Author information
Authors and Affiliations
Contributions
Q.H., Z.L., P.-L.T. and P.S. designed the research. Y.L. conducted material preparations and most of the characterization. H.S. and L.L. conducted the electrochemical tests and Z.L., P.-L.T. and P.S. analysed the data. H.S., B.D. and P.R. carried out the in situ XRD. J.L., P.O.Å.P, P.E. and L.H. carried out the STEM measurements. E.R.-P. performed the temperature-programmed desorption/mass spectrometry. Z.L., P.S. and Q.H. prepared the manuscript. All authors contributed to the discussion of the data and writing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, text, materials, Tables 1–8 and full reference list.
Source data
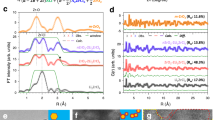
Source Data Fig. 2
Source Data for Fig. 2a,d.
Source Data Fig. 4
Source Data for Fig. 4.
Rights and permissions
About this article
Cite this article
Li, Y., Shao, H., Lin, Z. et al. A general Lewis acidic etching route for preparing MXenes with enhanced electrochemical performance in non-aqueous electrolyte. Nat. Mater. 19, 894–899 (2020). https://doi.org/10.1038/s41563-020-0657-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0657-0
This article is cited by
-
Nanofeather ruthenium nitride electrodes for electrochemical capacitors
Nature Materials (2024)
-
Capacitive tendency concept alongside supervised machine-learning toward classifying electrochemical behavior of battery and pseudocapacitor materials
Nature Communications (2024)
-
Comprehensive synthesis of Ti3C2Tx from MAX phase to MXene
Nature Protocols (2024)
-
One-step construction of strongly coupled Co3V2O8/Co3O4/MXene heterostructure via in-situ Co-F bonds for high performance all-solid-state asymmetric supercapacitors
Rare Metals (2024)
-
MXenes/CNTs-based hybrids: Fabrications, mechanisms, and modification strategies for energy and environmental applications
Nano Research (2024)