Abstract
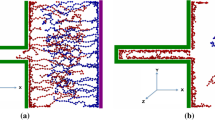
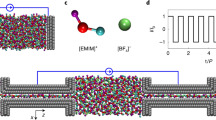
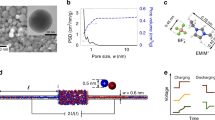
We performed constant-potential molecular dynamics simulations to analyse the double-layer structure and capacitive performance of supercapacitors composed of conductive metal–organic framework (MOF) electrodes and ionic liquids. The molecular modelling clarifies how ions transport and reside inside polarized porous MOFs, and then predicts the corresponding potential-dependent capacitance in characteristic shapes. The transmission line model was adopted to characterize the charging dynamics, which further allowed evaluation of the capacitive performance of this class of supercapacitors at the macroscale from the simulation-obtained data at the nanoscale. These ‘computational microscopy’ results were supported by macroscopic electrochemical measurements. Such a combined nanoscale-to-macroscale investigation demonstrates the potential of MOF supercapacitors for achieving unprecedentedly high volumetric energy and power densities. It gives molecular insights into preferred structures of MOFs for accomplishing consistent performance with optimal energy–power balance, providing a blueprint for future characterization and design of these new supercapacitor systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The theoretical and experimental data presented in this work are available from the corresponding authors on reasonable request.
References
Simon, P. & Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 7, 845–854 (2008).
Sun, H. et al. Hierarchical 3D electrodes for electrochemical energy storage. Nat. Rev. Mater. 4, 45–60 (2018).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal-organic frameworks. Science 341, 1230444 (2013).
Guan, B. Y., Yu, X. Y., Wu, H. B. & Lou, X. W. Complex nanostructures from materials based on metal–organic frameworks for electrochemical energy storage and conversion. Adv. Mater. 29, 1703614 (2017).
Wang, H., Zhu, Q.-L., Zou, R. & Xu, Q. Metal-organic frameworks for energy applications. Chem 2, 52–80 (2017).
Sun, L., Campbell, M. G. & Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 55, 3566–3579 (2016).
Zhou, J. & Wang, B. Emerging crystalline porous materials as a multifunctional platform for electrochemical energy storage. Chem. Soc. Rev. 46, 6927–6945 (2017).
Feng, D. et al. Robust and conductive two-dimensional metal–organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 3, 30–36 (2018).
Choi, K. M. et al. Supercapacitors of nanocrystalline metal–organic frameworks. ACS Nano 8, 7451–7457 (2014).
Sheberla, D. et al. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 16, 220–224 (2017).
Fedorov, M. V. & Kornyshev, A. A. Ionic liquids at electrified interfaces. Chem. Rev. 114, 2978–3036 (2014).
Hayes, R., Warr, G. G. & Atkin, R. Structure and nanostructure in ionic liquids. Chem. Rev. 115, 6357–6426 (2015).
Watanabe, M. et al. Application of ionic liquids to energy storage and conversion materials and devices. Chem. Rev. 117, 7190–7239 (2017).
Armand, M., Endres, F., MacFarlane, D. R., Ohno, H. & Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 8, 621–629 (2009).
Salanne, M. et al. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 1, 16070 (2016).
Vatamanu, J., Borodin, O., Olguin, M., Yushin, G. & Bedrov, D. Charge storage at the nanoscale: understanding the trends from the molecular scale perspective. J. Mater. Chem. A 5, 21049–21076 (2017).
Zhan, C. et al. Computational insights into materials and interfaces for capacitive energy storage. Adv. Sci. 4, 1700059 (2017).
Shao, Y. et al. Design and mechanisms of asymmetric supercapacitors. Chem. Rev. 118, 9233–9280 (2018).
Vatamanu, J., Borodin, O. & Smith, G. D. Molecular insights into the potential and temperature dependences of the differential capacitance of a room-temperature ionic liquid at graphite electrodes. J. Am. Chem. Soc. 132, 14825–14833 (2010).
Kornyshev, A. A. & Qiao, R. Three-dimensional double layers. J. Phys. Chem. C 118, 18285–18290 (2014).
Largeot, C. et al. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 130, 2730–2731 (2008).
Zhong, C. et al. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 44, 7484–7539 (2015).
Yang, X., Cheng, C., Wang, Y., Qiu, L. & Li, D. Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341, 534–537 (2013).
Yang, H. et al. Graphene supercapacitor with both high power and energy density. Nanotechnology 28, 445401 (2017).
Kondrat, S., Wu, P., Qiao, R. & Kornyshev, A. A. Accelerating charging dynamics in subnanometre pores. Nat. Mater. 13, 387–393 (2014).
Masarapu, C., Zeng, H. F., Hung, K. H. & Wei, B. Effect of temperature on the capacitance of carbon nanotube supercapacitors. ACS Nano 3, 2199–2206 (2009).
Fletcher, S. I. et al. The effects of temperature on the performance of electrochemical double layer capacitors. J. Power Sources 195, 7484–7488 (2010).
Stoppa, A., Zech, O., Kunz, W. & Buchner, R. The conductivity of imidazolium-based ionic liquids from (−35 to 195) °C. A. Variation of cation’s alkyl chain. J. Chem. Eng. Data 55, 1768–1773 (2010).
Kondrat, S. & Kornyshev, A. A. Superionic state in double-layer capacitors with nanoporous electrodes. J. Phys. Condens. Matter 23, 022201 (2011).
Futamura, R. et al. Partial breaking of the Coulombic ordering of ionic liquids confined in carbon nanopores. Nat. Mater. 16, 1225 (2017).
Merlet, C. et al. On the molecular origin of supercapacitance in nanoporous carbon electrodes. Nat. Mater. 11, 306–310 (2012).
Sheberla, D. et al. High electrical conductivity in Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2, a semiconducting metal–organic graphene analogue. J. Am. Chem. Soc. 136, 8859–8862 (2014).
Chaban, V. V., Voroshylova, I. V. & Kalugin, O. N. A new force field model for the simulation of transport properties of imidazolium-based ionic liquids. Phys. Chem. Chem. Phys. 13, 7910–7920 (2011).
Sun, L. et al. A microporous and naturally nanostructured thermoelectric metal-organic framework with ultralow thermal conductivity. Joule 1, 168–177 (2017).
Park, J. et al. Stabilization of hexaaminobenzene in a 2D conductive metal–organic framework for high power sodium storage. J. Am. Chem. Soc. 140, 10315–10323 (2018).
Nonoguchi, Y., Sato, D. & Kawai, T. Crystallinity-dependent thermoelectric properties of a two-dimensional coordination polymer: Ni3(2,3,6,7,10,11-hexaiminotriphenylene)2. Polymers 10, 962 (2018).
Izadi-Najafabadi, A., Futaba, D. N., Iijima, S. & Hata, K. Ion diffusion and electrochemical capacitance in aligned and packed single-walled carbon nanotubes. J. Am. Chem. Soc. 132, 18017–18019 (2010).
Eftekhari, A. Supercapacitors utilising ionic liquids. Energy Storage Mater. 9, 47–69 (2017).
González, A., Goikolea, E., Barrena, J. A. & Mysyk, R. Review on supercapacitors: technologies and materials. Renew. Sustain. Energy Rev. 58, 1189–1206 (2016).
Li, P. & Wang, B. Recent development and application of conductive MOFs. Isr. J. Chem. 58, 1010–1018 (2018).
Vatamanu, J., Vatamanu, M. & Bedrov, D. Non-faradaic energy storage by room temperature ionic liquids in nanoporous electrodes. ACS Nano 9, 5999–6017 (2015).
Park, J. et al. Synthetic routes for a 2D semiconductive copper hexahydroxybenzene metal–organic framework. J. Am. Chem. Soc. 140, 14533–14537 (2018).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Rappe, A. K., Casewit, C. J., Colwell, K. S., Goddard, W. A. & Skiff, W. M. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 4, 435–447 (2008).
Bussi, G., Donadio, D. & Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 126, 014101 (2007).
Gingrich, T. R. & Wilson, M. On the Ewald summation of Gaussian charges for the simulation of metallic surfaces. Chem. Phys. Lett. 500, 178–183 (2010).
Fang, T., Konar, A., Xing, H. & Jena, D. Carrier statistics and quantum capacitance of graphene sheets and ribbons. Appl. Phys. Lett. 91, 092109–092103 (2007).
Xia, J., Chen, F., Li, J. & Tao, N. Measurement of the quantum capacitance of graphene. Nat. Nanotechnol. 4, 505–509 (2009).
Zhan, C., Neal, J., Wu, J. & Jiang, D. Quantum effects on the capacitance of graphene-based electrodes. J. Phys. Chem. C 119, 22297–22303 (2015).
Acknowledgements
G.F., S.B., Ming Chen, L.N., Mingyu Chen, T.W., J.W., R.W. and J.F. acknowledge the funding support from the National Natural Science Foundation of China (51876072, 51836003) and Shenzhen Basic Research Project (JCYJ20170307171511292). S.B. and R.W. thank the China Scholarship Council for financial support. A.A.K. acknowledges the Leverhulme Trust for funding (RPG-2016-223), HUST for the support of this project through the HUST Honorary Professorship, and Imperial College for the support of this form of collaboration between the involved HUST and Imperial groups, and thanks the Imperial College–MIT seed fund for supporting the collaboration between the two universities. M.D., H.B. and T.C. thank the Army Research Office (W911NF-17-1-0174) for support. The computation was completed using the Tianhe II supercomputer in the National Supercomputing Center in Guangzhou. Part of the characterization was performed at the Analytical & Testing Center of HUST.
Author information
Authors and Affiliations
Contributions
G.F. and A.A.K. set the strategy of this project in consultation with M.D.; G.F. devised simulation approaches; G.F. and M.D. designed the experiment. S.B. performed the major part of the MD simulations with participation of Ming Chen, R.W. and J.F.; Ming Chen did all density functional theory calculations; H.B., L.N., Mingyu Chen, T.W., J.W. and T.C. carried out the experiment, in which L.N. developed MOF synthesis procedures; G.F., S.B., Ming Chen, L.N., Mingyu Chen and A.A.K. analysed the data and wrote the manuscript; G.F., S.B., A.A.K., Ming Chen, H.B. and M.D. contributed to the discussion of results, editing and revising the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29, discussion and Tables 1–6.
Rights and permissions
About this article
Cite this article
Bi, S., Banda, H., Chen, M. et al. Molecular understanding of charge storage and charging dynamics in supercapacitors with MOF electrodes and ionic liquid electrolytes. Nat. Mater. 19, 552–558 (2020). https://doi.org/10.1038/s41563-019-0598-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0598-7
This article is cited by
-
Crystallization of molecular layers produced under confinement onto a surface
Nature Communications (2024)
-
Imaging the dynamic influence of functional groups on metal-organic frameworks
Nature Communications (2023)
-
Review of the role of ionic liquids in two-dimensional materials
Frontiers of Physics (2023)
-
Molecular Understanding of Heat Transfer in Ionic-Liquid-based Electric Double Layers
Journal of Thermal Science (2023)
-
MOF-derived spherical trimetallic NiZnCo-LDH for hybrid supercapacitors
Ionics (2023)