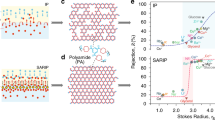
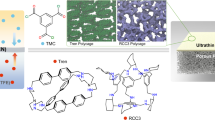
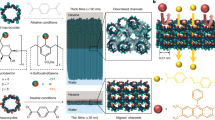
Abstract
Biological membranes are ideal for separations as they provide high permeability while maintaining high solute selectivity due to the presence of specialized membrane protein (MP) channels. However, successful integration of MPs into manufactured membranes has remained a significant challenge. Here, we demonstrate a two-hour organic solvent method to develop 2D crystals and nanosheets of highly packed pore-forming MPs in block copolymers (BCPs). We then integrate these hybrid materials into scalable MP-BCP biomimetic membranes. These MP-BCP nanosheet membranes maintain the molecular selectivity of the three types of β-barrel MP channels used, with pore sizes of 0.8 nm, 1.3 nm, and 1.5 nm. These biomimetic membranes demonstrate water permeability that is 20–1,000 times greater than that of commercial membranes and 1.5–45 times greater than that of the latest research membranes with comparable molecular exclusion ratings. This approach could provide high performance alternatives in the challenging sub-nanometre to few-nanometre size range.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its supplementary information files and available from the authors upon reasonable request.
References
Kumar, M., Grzelakowski, M., Zilles, J., Clark, M. & Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl Acad. Sci. USA 104, 20719–20724 (2007).
Ren, T. W. et al. Membrane protein insertion into and compatibility with biomimetic membranes. Adv. Biosyst. 1, 1700053 (2017).
Kumar, M., Habel, J. E., Shen, Y.-X., Meier, W. P. & Walz, T. High-density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J. Am. Chem. Soc. 134, 18631–18637 (2012).
Chowdhury, R. et al. PoreDesigner for tuning solute selectivity in a robust and highly permeable outer membrane pore. Nat. Commun. 9, 3661 (2018).
Shen, Y.-x et al. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl Acad. Sci. USA 112, 9810–9815 (2015).
Kocsis, I., Sun, Z., Legrand, Y. M. & Barboiu, M. Artificial water channels—deconvolution of natural Aquaporins through synthetic design. npj Clean Water 1, 13 (2018).
Shen, Y.-x et al. Achieving high permeability and enhanced selectivity for Angstrom-scale separations using artificial water channel membranes. Nat. Commun. 9, 2294 (2018).
Lang, C. et al. Biomimetic separation of transport and matrix functions in lamellar block copolymer channel-based membranes. ACS Nano 13, 8292–8302 (2019).
Tunuguntla, R. H. et al. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 357, 792–796 (2017).
Park, H. B., Kamcev, J., Robeson, L. M., Elimelech, M. & Freeman, B. D. Maximizing the right stuff: the trade-off between membrane permeability and selectivity. Science 356, eaab0530 (2017).
Mohammad, A. W. et al. Nanofiltration membranes review: recent advances and future prospects. Desalination 356, 226–254 (2015).
Shen, Y. X., Saboe, P. O., Sines, I. T., Erbakan, M. & Kumar, M. Biomimetic membranes: a review. J. Membr. Sci. 454, 359–381 (2014).
Duong, P. H. H. et al. Planar biomimetic aquaporin-incorporated triblock copolymer membranes on porous alumina supports for nanofiltration. J. Membr. Sci. 409, 34–43 (2012).
Wang, M. et al. Layer-by-layer assembly of aquaporin Z-incorporated biomimetic membranes for water purification. Environ. Sci. Technol. 49, 3761–3768 (2015).
Helix-Nielsen, C. Biomimetic membranes as a technology platform: challenges and opportunities. Membranes 8, 44 (2018).
Tang, C. Y., Zhao, Y., Wang, R., Helix-Nielsen, C. & Fane, A. G. Desalination by biomimetic aquaporin membranes: review of status and prospects. Desalination 308, 34–40 (2013).
Song, W., Tu, Y.-M., Oh, H., Samineni, L. & Kumar, M. Hierarchical optimization of high-performance biomimetic and bioinspired membranes. Langmuir 35, 589–607 (2018).
Tamm, L. K., Arora, A. & Kleinschmidt, J. H. Structure and assembly of beta-barrel membrane proteins. J. Biol. Chem. 276, 32399–32402 (2001).
Wimley, W. C. The versatile β-barrel membrane protein. Curr. Opin. Struct. Biol. 13, 404–411 (2003).
Cowan, S. W. et al. Crystal structures explain functional properties of two E. coli porins. Nature 358, 727–733 (1992).
Phale, P. S. et al. Stability of trimeric OmpF porin: the contributions of the latching loop L2. Biochemistry 37, 15663–15670 (1998).
Lee, A. G. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta 1612, 1–40 (2003).
Palivan, C. G. et al. Bioinspired polymer vesicles and membranes for biological and medical applications. Chem. Soc. Rev. 45, 377–411 (2016).
Klara, S. S. et al. Magnetically directed two-dimensional crystallization of OmpF membrane proteins in block copolymers. J. Am. Chem. Soc. 138, 28–31 (2016).
Cowan, S. et al. The structure of OmpF porin in a tetragonal crystal form. Structure 3, 1041–1050 (1995).
Pebay-Peyroula, E., Garavito, R., Rosenbusch, J., Zulauf, M. & Timmins, P. Detergent structure in tetragonal crystals of OmpF porin. Structure 3, 1051–1059 (1995).
Haltia, T. & Freire, E. Forces and factors that contribute to the structural stability of membrane-proteins. Biochim. Biophys. Acta 1228, 1–27 (1995).
White, S. H. & Wimley, W. C. Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28, 319–365 (1999).
Kleffel, B., Garavito, R. M., Baumeister, W. & Rosenbusch, J. P. Secondary structure of a channel-forming protein: porin from E. coli outer membranes. EMBO J. 4, 1589–1592 (1985).
Mohammad, M. M., Howard, K. R. & Movileanu, L. Redesign of a plugged β-barrel membrane protein. J. Biol. Chem. 286, 8000–8013 (2011).
Gouaux, J. E. et al. Subunit stoichiometry of staphylococcal alpha-hemolysin in crystals and on membranes: a heptameric transmembrane pore. Proc. Natl Acad. Sci. USA 91, 12828–12831 (1994).
Jap, B. K. et al. 2D crystallization: from art to science. Ultramicroscopy 46, 45–84 (1992).
Perry, M. et al. Challenges in commercializing biomimetic membranes. Membranes 5, 685–701 (2015).
Kowal, J., Zhang, X. Y., Dinu, I. A., Palivan, C. G. & Meier, W. Planar biomimetic membranes based on amphiphilic block copolymers. ACS Macro Lett. 3, 59–63 (2014).
Jin, H. et al. Highly stable and self-repairing membrane-mimetic 2D nanomaterials assembled from lipid-like peptoids. Nat. Commun. 7, 12252 (2016).
Biyani, N. et al. Focus: the interface between data collection and data processing in cryo-EM. J. Struct. Biol. 198, 124–133 (2017).
Signorell, G. A., Kaufmann, T. C., Kukulski, W., Engel, A. & Rémigy, H.-W. Controlled 2D crystallization of membrane proteins using methyl-β-cyclodextrin. J. Struct. Biol. 157, 321–328 (2007).
Ferro, M. et al. Organic solvent extraction as a versatile procedure to identify hydrophobic chloroplast membrane proteins. Electrophoresis 21, 3517–3526 (2000).
Panganiban, B. et al. Random heteropolymers preserve protein function in foreign environments. Science 359, 1239–1243 (2018).
Huang, H. B., Ying, Y. L. & Peng, X. S. Graphene oxide nanosheet: an emerging star material for novel separation membranes. J. Mater. Chem. A 2, 13772–13782 (2014).
Peng, Y. et al. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 346, 1356–1359 (2014).
Liu, G., Jin, W. & Xu, N. Two-dimensional-material membranes: a new family of high-performance separation membranes. Angew. Chem. Int. Edit. 55, 13384–13397 (2016).
Nikaido, H. & Saier, M. H. Transport proteins in bacteria: common themes in their design. Science 258, 936–942 (1992).
Niedzwiecki, D. J., Mohammad, M. M. & Movileanu, L. Inspection of the engineered FhuA ΔC/Δ4L protein nanopore by polymer exclusion. Biophys. J. 103, 2115–2124 (2012).
Bezrukov, S. M., Vodyanoy, I., Brutyan, R. A. & Kasianowicz, J. J. Dynamics and free energy of polymers partitioning into a nanoscale pore. Macromolecules 29, 8517–8522 (1996).
Zhang, R. et al. Antifouling membranes for sustainable water purification: strategies and mechanisms. Chem. Soc. Rev. 45, 5888–5924 (2016).
Jang, E.-S. et al. Influence of concentration polarization and thermodynamic non-ideality on salt transport in reverse osmosis membranes. J. Membr. Sci. 572, 668–675 (2019).
Kyte, J. & Doolittle, R. F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 157, 105–132 (1982).
Jegal, J., Min, S. G. & Lee, K. H. Factors affecting the interfacial polymerization of polyamide active layers for the formation of polyamide composite membranes. J. Appl. Polym. Sci. 86, 2781–2787 (2002).
Dey, K. et al. Selective molecular separation by interfacially crystallized covalent organic framework thin films. J. Am. Chem. Soc. 139, 13083–13091 (2017).
Han, G., Feng, Y., Chung, T.-S., Weber, M. & Maletzko, C. Phase inversion directly induced tight ultrafiltration (UF) hollow fiber membranes for effective removal of textile dyes. Environ. Sci. Technol. 51, 14254–14261 (2017).
Gessmann, D. et al. Improving the resistance of a eukaryotic β-barrel protein to thermal and chemical perturbations. J. Mol. Biol. 413, 150–161 (2011).
Hammill, J. T., Miyake-Stoner, S., Hazen, J. L., Jackson, J. C. & Mehl, R. A. Preparation of site-specifically labeled fluorinated proteins for 19 F-NMR structural characterization. Nat. Protoc. 2, 2601–2607 (2007).
Spurr, A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43 (1969).
Adams, M. H. Bacteriophages (Interscience, 1959).
Perunov, N. & England, J. L. Quantitative theory of hydrophobic effect as a driving force of protein structure. Protein Sci. 23, 387–399 (2014).
Kister, A. E. & Phillips, J. C. A stringent test for hydrophobicity scales: two proteins with 88% sequence identity but different structure and function. Proc. Natl Acad. Sci. USA 105, 9233–9237 (2008).
Acknowledgements
The authors acknowledge financial support from the National Science Foundation (NSF) CAREER grant (CBET-1552571), NSF grant CBET-1709522 and NSF grant CBET-1804836 to M.K. for this work. T.C. and E.D.G. acknowledge financial support from NSF DMR-1609417. The authors also thank M. Hazen and J. Cantolina for their help with cross-sectional sample preparation. We thank L. Movileanu for the kind gift of the plasmid for expressing the FhuA ΔC/Δ4L protein.
Author information
Authors and Affiliations
Contributions
Y.-M.T., W.S., T.R. and M.K. conceived and designed the research. T.R., W.S., Y.-M.T., Y.-x.S. P.R., T.E.C. and L.S. performed the experiments, with the assistance of R.C., C.L., A.T., D.C., Y.D., A.M. and M.Z. in specialized analytic tools. T.R., Y.-M.T., P.R., A.P., J.N.S., S.H.M. and M.G. contributed to the protein production. Y.-M.T., W.S., T.R., R.C., T.E.C., L.S., D.B., W.A.P., E.D.G., R.J.H., Y.W. and M.K. analysed and interpreted the data and results. T.R., W.S., Y.-M.T. and M.K. co-wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A.P. and M.G. are employed by Applied Biomimetic, which aims to commercialize biomimetic membranes similar to those presented in this Article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, Tables 1–5, Figures 1–19, references 1–31.
Rights and permissions
About this article
Cite this article
Tu, YM., Song, W., Ren, T. et al. Rapid fabrication of precise high-throughput filters from membrane protein nanosheets. Nat. Mater. 19, 347–354 (2020). https://doi.org/10.1038/s41563-019-0577-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0577-z
This article is cited by
-
Effective pathogen removal in sustainable natural fiber Moringa filters
npj Clean Water (2022)
-
Nanostructured block copolymer muscles
Nature Nanotechnology (2022)
-
A bibliometric study on biomimetic and bioinspired membranes for water filtration
npj Clean Water (2021)
-
Artificial channels for confined mass transport at the sub-nanometre scale
Nature Reviews Materials (2021)
-
Bioinspired and biomimetic membranes for water purification and chemical separation: A review
Frontiers of Environmental Science & Engineering (2021)