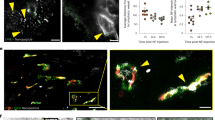
Abstract
The concept of nanoparticle transport through gaps between endothelial cells (inter-endothelial gaps) in the tumour blood vessel is a central paradigm in cancer nanomedicine. The size of these gaps was found to be up to 2,000 nm. This justified the development of nanoparticles to treat solid tumours as their size is small enough to extravasate and access the tumour microenvironment. Here we show that these inter-endothelial gaps are not responsible for the transport of nanoparticles into solid tumours. Instead, we found that up to 97% of nanoparticles enter tumours using an active process through endothelial cells. This result is derived from analysis of four different mouse models, three different types of human tumours, mathematical simulation and modelling, and two different types of imaging techniques. These results challenge our current rationale for developing cancer nanomedicine and suggest that understanding these active pathways will unlock strategies to enhance tumour accumulation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the annotated and analysed TEM images are uploaded on the Figshare server. This includes an Excel sheet that summarizes the results and overall analysis of the TEM images. This is available at https://doi.org/10.6084/m9.figshare.7485770. 3D images used in the simulations are also stored on Figshare and will be automatically downloaded by the code used for simulations. All other datasets generated and analysed during this study are available from the corresponding author upon reasonable request.
Code availability
All code for simulations of nanoparticles in tumours can be found at https://github.com/jbRothschild/nano-extravasation.
The code for spatial analysis of nanoparticles is uploaded to Figshare at https://doi.org/10.6084/m9.figshare.7485770.
References
Wilhelm, S. et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 1, 16014 (2016).
Jain, R. K. Transport of molecules, particles, and cells in solid tumors. Annu. Rev. Biomed. Eng. 1, 241–263 (1999).
Hobbs, S. K. et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl Acad. Sci. USA 95, 4607–4612 (1998).
Jain, R. K. & Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010).
Hashizume, H. et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 156, 1363–1380 (2000).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2, 751–760 (2007).
Gerlowski, L. E. & Jain, R. K. Microvascular permeability of normal and neoplastic tissues. Microvasc. Res. 31, 288–305 (1986).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 46, 6387–6392 (1986).
Nichols, J. W. & Bae, Y. H. EPR: evidence and fallacy. J. Control. Release 190, 451–464 (2014).
Danhier, F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 244, 108–121 (2016).
Kim, S. M., Faix, P. H. & Schnitzer, J. E. Overcoming key biological barriers to cancer drug delivery and efficacy. J. Control. Release 267, 15–30 (2017).
Nakamura, Y., Mochida, A., Choyke, P. L. & Kobayashi, H. Nanodrug delivery: is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 27, 2225–2238 (2016).
Nel, A., Ruoslahti, E. & Meng, H. New insights into ‘permeability’ as in the enhanced permeability and retention effect of cancer nanotherapeutics. ACS Nano 11, 9567–9569 (2017).
Huynh, E. & Zheng, G. Cancer nanomedicine: addressing the dark side of the enhanced permeability and retention effect. Nanomedicine 10, 1993–1995 (2015).
Nakamura, H., Jun, F. & Maeda, H. Development of next-generation macromolecular drugs based on the EPR effect: challenges and pitfalls. Expert Opin. Drug Deliv. 12, 53–64 (2015); erratum 12, 691 (2015).
Rosenblum, D., Joshi, N., Tao, W., Karp, J. M. & Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 9, 1410 (2018).
Chan, W. C. W. Nanomedicine 2.0. Acc. Chem. Res. 50, 627–632 (2017).
Nagy, J. A. et al. Permeability properties of tumor surrogate blood vessels induced by VEGF-A. Lab. Invest. 86, 767–780 (2006).
Feng, D. et al. Reinterpretation of endothelial cell gaps induced by vasoactive mediators in guinea-pig, mouse and rat: many are transcellular pores. J. Physiol. 504, 747–761 (1997).
Feng, D. et al. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation 6, 23–44 (1999).
Dvorak, H. F., Nagy, J. A., Dvorak, J. T. & Dvorak, A. M. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am. J. Pathol. 133, 95–109 (1988).
Feng, D., Nagy, J. A., Dvorak, H. F. & Dvorak, A. M. Ultrastructural studies define soluble macromolecular, particulate, and cellular transendothelial cell pathways in venules, lymphatic vessels, and tumor-associated microvessels in man and animals. Microsc. Res. Tech. 57, 289–326 (2002).
Neal, C. R. & Michel, C. C. Transcellular openings through frog microvascular endothelium. Exp. Physiol. 82, 419–422 (1997).
Michel, C. C. & Neal, C. R. Openings through endothelial cells associated with increased microvascular permeability. Microcirculation 6, 45–54 (1999).
Syed, A. M. et al. Three-dimensional imaging of transparent tissues via metal nanoparticle labeling. J. Am. Chem. Soc. 139, 9961–9971 (2017).
Sindhwani, S., Syed, A. M., Wilhelm, S. & Chan, W. C. W. Exploring passive clearing for 3D optical imaging of nanoparticles in intact tissues. Bioconjug. Chem. 28, 253–259 (2017).
Sindhwani, S. et al. Three-dimensional optical mapping of nanoparticle distribution in intact tissues. ACS Nano 10, 5468–5478 (2016).
Ramanujan, S. et al. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 83, 1650–1660 (2002).
Sykes, E. A. et al. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl Acad. Sci. USA 113, E1142–E1151 (2016).
Schnitzer, J. E. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am. J. Physiol. Heart Circulatory Physiol. 262, H246–H254 (1992).
Schnitzer, J. E. in Whole Organ Approaches to Cellular Metabolism (eds Bassingthwaighte, J., Goresky, C. A., Linehan, J. H 31–69 (Springer, 1998).
Oh, P. et al. In vivo proteomic imaging analysis of caveolae reveals pumping system to penetrate solid tumors. Nat. Med. 20, 1062–1068 (2014).
Thurston, G. et al. Cationic liposomes target angiogenic endothelial cells in tumors and chronic inflammation in mice. J. Clin. Invest. 101, 1401–1413 (1998).
McDonald, D. M. Uptake of cationic liposomes by normal and angiogenic endothelial cells in vivo. Nat. Biotechnol. 17, 14–14 (1999).
Tkachenko, E. et al. Caveolae, fenestrae and transendothelial channels retain PV1 on the surface of endothelial cells. PLoS ONE 7, e32655 (2012).
Stan, R. V., Tkachenko, E. & Niesman, I. R. PV1 Is a key structural component for the formation of the stomatal and fenestral diaphragms. Mol. Biol. Cell 15, 3615–3630 (2004).
Stan, R. V. et al. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev. Cell 23, 1203–1218 (2012).
Dai, Q. et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 12, 8423–8435 (2018).
Qaddoumi, M. G. et al. Clathrin and caveolin-1 expression in primary pigmented rabbit conjunctival epithelial cells: role in PLGA nanoparticle endocytosis. Mol. Vis. 9, 559–568 (2003).
Voigt, J., Christensen, J. & Shastri, V. P. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc. Natl Acad. Sci. USA 111, 2942–2947 (2014).
Ho, Y. T., Kamm, R. D. & Kah, J. C. Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 10, 12386–12397 (2018).
Chauhan, V. P. et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat. Nanotechnol. 7, 383–388 (2012).
Schubert, W. et al. Caveolae-deficient endothelial cells show defects in the uptake and transport of albumin in vivo. J. Biol. Chem. 276, 48619–48622 (2001).
Matsumoto, Y. et al. Vascular bursts enhance permeability of tumour blood vessels and improve nanoparticle delivery. Nat. Nanotechnol. 11, 533–538 (2016).
Naumenko, V. A. et al. Extravasating neutrophils open vascular barrier and improve liposomes delivery to tumors. ACS Nano 13, 12599–12612 (2019).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov. 5, 932–943 (2015).
Perrault, S. D. & Chan, W. C. W. Synthesis and surface modification of highly monodispersed, spherical gold nanoparticles of 50-200 nm. J. Am. Chem. Soc. 131, 17042–17043 (2009).
Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 241, 20–22 (1973).
Sykes, E. A., Chen, J., Zheng, G. & Chan, W. C. W. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano 8, 5696–5706 (2014).
Nakasone, E. S. et al. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21, 488–503 (2012).
Egeblad, M. et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis. Model. Mech. 1, 155–167 (2008). discussion 165.
Chou, L. Y. T. & Chan, W. C. W. Fluorescence-tagged gold nanoparticles for rapidly characterizing the size-dependent biodistribution in tumor models. Adv. Healthc. Mater. 1, 714–721 (2012).
Tavares, A. J. et al. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc. Natl Acad. Sci. U. S. A. 114, E10871–E10880 (2017).
Acknowledgements
The authors thank D. Holmyard and A. Darbandi at SickKids Hospital (Toronto) for their help in preparing tissue grids for TEM. The authors also thank the Ontario Tumour Bank (Canada) for cooperation with the sample collection from human biopsy samples. The Canadian authors also thank the Canadian Research Chairs Program (950-223924), Canadian Cancer Society (502200 and 706286), Natural Sciences and Engineering Research Council (2015-06397 and graduate fellowships), Walter C. Sumner Memorial Fellowship (graduate fellowships), and Canadian Institute of Health Research (PJT-148848 and FDN-159932, and graduate fellowships) for funding support. M.E. thanks the Department of Defence (DoD) for grant W81XWH-14-1-0078 and is a BCRP-Era of Hope Scholar. L.M. thanks Northwell Health for funding.
Author information
Authors and Affiliations
Contributions
S.S., A.M.S. and W.C.W.C. conceived the idea, S.S., A.M.S., J.N., B.R.K., L.M., J.L.Y.W., S.W., B.O. and Z.L. performed the experiments. S.S., A.M.S., J.N., B.R.K., P.M., Y.Z., N.U.R. and T.H. annotated the TEM images. A.M.S., J.R. and A.Z. performed the computational simulations and analysis. S.G., A.S. and L.W. provided the PDX model. L.M. and M.E. provided the GEMM model. S.S., A.M.S., J.N., B.R.K., M.E. and W.C.W.C. prepared and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Tables 1–13, Notes 1 and 2, Discussion and References.
Supplementary Video 1
The video recording of the fixation and nanoparticle circulation procedure for Zombie model has been attached. Mouse was fixed via transcardial perfusion. Known volume and concentration of nanoparticles were added into the perfusion chamber and the nanoparticles were perfused throughout the fixed mouse with a peristaltic pump.
Supplementary Video 2a
Time lapse videos from intravital imaging of 4T1 tumour model showing that nanoparticles form “hotspots” along the vessels. These sites of extravasation are colocalizing with the vessels. These hotspots are heterogenous in their distribution across the field of view.
Supplementary Video 2b
Time lapse videos from intravital imaging of MMTV-PyMT tumour model showing that nanoparticles form “hotspots” along the vessels similar to 4T1 model in Video S2a.
Supplementary Video 3a
Video rendering of 3D images of human breast tumour immunolabeled for V-Cadherin and PV-1.
Supplementary Video 3b
Video rendering of 3D images of human ovarian tumour immunolabeled for V-Cadherin and PV-1.
Supplementary Video 3c
Video rendering of 3D images of human brain tumour immunolabeled for V-Cadherin and PV-1.
Rights and permissions
About this article
Cite this article
Sindhwani, S., Syed, A.M., Ngai, J. et al. The entry of nanoparticles into solid tumours. Nat. Mater. 19, 566–575 (2020). https://doi.org/10.1038/s41563-019-0566-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0566-2
This article is cited by
-
Forefronts and hotspots evolution of the nanomaterial application in anti-tumor immunotherapy: a scientometric analysis
Journal of Nanobiotechnology (2024)
-
Strategies for non-viral vectors targeting organs beyond the liver
Nature Nanotechnology (2024)
-
Anti-lymphangiogenesis for boosting drug accumulation in tumors
Signal Transduction and Targeted Therapy (2024)
-
Nucleic acid-based drugs for patients with solid tumours
Nature Reviews Clinical Oncology (2024)
-
Entry and exit of extracellular vesicles to and from the blood circulation
Nature Nanotechnology (2024)