Abstract
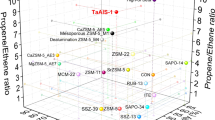
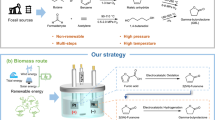
The efficient production of light olefins from renewable biomass is a vital and challenging target to achieve future sustainable chemical processes. Here we report a hetero-atomic MFI-type zeolite (NbAlS-1), over which aqueous solutions of γ-valerolactone (GVL), obtained from biomass-derived carbohydrates, can be quantitatively converted into butenes with a yield of >99% at ambient pressure under continuous flow conditions. NbAlS-1 incorporates simultaneously niobium(v) and aluminium(iii) centres into the framework and thus has a desirable distribution of Lewis and Brønsted acid sites with optimal strength. Synchrotron X-ray diffraction and absorption spectroscopy show that there is cooperativity between Nb(v) and the Brønsted acid sites on the confined adsorption of GVL, whereas the catalytic mechanism for the conversion of the confined GVL into butenes is revealed by in situ inelastic neutron scattering, coupled with modelling. This study offers a prospect for the sustainable production of butene as a platform chemical for the manufacture of renewable materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the relevant data are available from the authors, and/or are included with the manuscript.
References
Torres Galvis, H. M. & de Jong, K. P. Catalysts for production of lower olefins from synthesis gas: a review. ACS Catal. 3, 2130–2149 (2013).
Bender, M. An overview of industrial processes for the production of olefins—C4 hydrocarbons. ChemBioEng Rev. 1, 136–147 (2014).
Galvis, H. M. T. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 335, 835–838 (2012).
Amghizar, I., Vandewalle, L. A., Van Geem, K. M. & Marin, G. B. New trends in olefin production. Engineering 3, 171–178 (2017).
Jiao, F. et al. Selective conversion of syngas to light olefins. Science 351, 1065–1068 (2016).
Zacharopoulou, V. & Lemonidou, A. A. Olefins from biomass intermediates: a review. Catalysts 8, 2 (2018).
Tuck, C. O., Pérez, E., Horváth, I. T., Sheldon, R. A. & Poliakoff, M. Valorization of biomass: deriving more value from waste. Science 337, 695–699 (2012).
Bozell, J. J. Connecting biomass and petroleum processing with a chemical bridge. Science 329, 522–523 (2010).
Bond, J. Q., Alonso, D. M., Wang, D., West, R. M. & Dumesic, J. A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 327, 1110–1114 (2010).
Jing, Y., Guo, Y., Xia, Q., Liu, X. & Wang, Y. Catalytic production of value-added chemicals and liquid fuels from lignocellulosic biomass. Chem 5, 2520–2546 (2019).
Corma, A., Iborra, S. & Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007).
Yan, K., Yang, Y., Chai, J. & Lu, Y. Catalytic reactions of gamma-valerolactone: a platform to fuels and value-added chemicals. Appl. Catal. B 179, 292–304 (2015).
Lin, L. et al. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 309, 136–145 (2014).
Hong, E., Park, J.-H. & Shin, C.-H. Oxidative dehydrogenation of n-butenes to 1,3-butadiene over bismuth molybdate and ferrite catalysts: a review. Catal. Surv. Asia 20, 23–33 (2016).
Ye, L. et al. Decarboxylation of lactones over Zn/ZSM-5: elucidation of the structure of the active site and molecular interactions. Angew. Chem. Int. Ed. 56, 10711–10716 (2017).
Shao, H., Lv, Z., Sun, Z., Liu, C. & He, A. Synthesis of spherical polyethylene/poly(1-butene) reactor blends with two-stage sequence polymerization technology. Polymer 144, 72–79 (2018).
Kim, M. S., Park, M. S., Seo, H. J. & Lee, S. H. Method for preparing polybutene. US Patent 9683060B2 (2017).
Kellicutt, A. B., Salary, R., Abdelrahman, O. A. & Bond, J. Q. An examination of the intrinsic activity and stability of various solid acids during the catalytic decarboxylation of γ-valerolactone. Catal. Sci. Technol. 4, 2267–2279 (2014).
Bond, J. Q., Wang, D., Alonso, D. M. & Dumesic, J. A. Interconversion between γ-valerolactone and pentenoic acid combined with decarboxylation to form butene over silica/alumina. J. Catal. 281, 290–299 (2011).
Bond, J. Q., Martin Alonso, D., West, R. M. & Dumesic, J. A. γ-Valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. Langmuir 26, 16291–16298 (2010).
Bond, J. Q., Jungong, C. S. & Chatzidimitriou, A. Microkinetic analysis of ring opening and decarboxylation of γ-valerolactone over silica alumina. J. Catal. 344, 640–656 (2016).
Yun, G.-N., Ahn, S.-J., Takagaki, A., Kikuchi, R. & Oyama, S. T. Hydrodeoxygenation of γ-valerolactone on bimetallic NiMo phosphide catalysts. J. Catal. 353, 141–151 (2017).
Lin, W.-C. et al. Zinc-incorporated microporous molecular sieve for mild catalytic hydrolysis of γ-valerolactone: a new selective route for biomass conversion. ChemSusChem 11, 4214–4218 (2018).
Serrano-Ruiz, J. C., Braden, D. J., West, R. M. & Dumesic, J. A. Conversion of cellulose to hydrocarbon fuels by progressive removal of oxygen. Appl. Catal. B 100, 184–189 (2010).
Okuhara, T. Water-tolerant solid acid catalysts. Chem. Rev. 102, 3641–3666 (2002).
Nakajima, K. et al. Nb2O5·nH2O as a heterogeneous catalyst with water-tolerant Lewis acid sites. J. Am. Chem. Soc. 133, 4224–4227 (2011).
Zhang, Y. et al. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurfural over water-tolerant niobium-based catalysts. Fuel 139, 301–307 (2015).
Zhang, Y. et al. Mesoporous niobium phosphate: an excellent solid acid for the dehydration of fructose to 5-hydroxymethylfurfural in water. Catal. Sci. Technol. 2, 2485–2491 (2012).
Takagaki, A., Tagusagawa, C. & Domen, K. Glucose production from saccharides using layered transition metal oxide and exfoliated nanosheets as a water-tolerant solid acid catalyst. Chem. Commun. 14, 5363–5365 (2008).
Carniti, P., Gervasini, A., Biella, S. & Auroux, A. Niobic acid and niobium phosphate as highly acidic viable catalysts in aqueous medium: fructose dehydration reaction. Catal. Today 118, 373–378 (2006).
Carniti, P., Gervasini, A., Bossola, F. & Dal Santo, V. Cooperative action of Brønsted and Lewis acid sites of niobium phosphate catalysts for cellobiose conversion in water. Appl. Catal. B 193, 93–102 (2016).
Nowak, I. & Ziolek, M. Niobium compounds: preparation, characterization, and application in heterogeneous catalysis. Chem. Rev. 99, 3603–3624 (1999).
Serrano-Ruiz, J. C., Wang, D. & Dumesic, J. A. Catalytic upgrading of levulinic acid to 5-nonanone. Green. Chem. 12, 574–577 (2010).
Xia, Q. et al. Direct hydrodeoxygenation of raw woody biomass into liquid alkanes. Nat. Commun. 7, 11162 (2016).
Shao, Y. et al. Selective production of arenes via direct lignin upgrading over a niobium-based catalyst. Nat. Commun. 8, 16104 (2017).
Prakash, A. M. & Kevan, L. Synthesis of niobium silicate molecular sieves of the MFI structure: evidence for framework incorporation of the niobium ion. J. Am. Chem. Soc. 120, 13148–13155 (1998).
Wichterlová, B., Nováková, J. & Prášil, Z. Structure of defects in γ-irradiated ZSM-5 and Y zeolites: an ESR study. Zeolites 8, 117–121 (1988).
Corma, A., Llabrés i Xamena, F. X., Prestipino, C., Renz, M. & Valencia, S. Water resistant, catalytically active Nb and Ta isolated Lewis acid sites, homogeneously distributed by direct synthesis in a beta zeolite. J. Phys. Chem. C 113, 11306–11315 (2009).
Duereh, A., Sato, Y., Smith, R. L. & Inomata, H. Analysis of the cybotactic region of two renewable lactone–water mixed-solvent systems that exhibit synergistic Kamlet–Taft basicity. J. Phys. Chem. B 120, 4467–4481 (2016).
Lemishko, T., Valencia, S., Rey, F., Jiménez-Ruiz, M. & Sastre, G. Inelastic neutron scattering study on the location of Brønsted acid sites in high silica LTA zeolite. J. Phys. Chem. C 120, 24904–24909 (2016).
Lemishko, T. et al. Inelastic neutron scattering study of the aluminum and Brønsted site location in aluminosilicate LTA zeolites. J. Phys. Chem. C 122, 11450–11454 (2018).
Hawkins, A. P. et al. Investigation of the dynamics of 1-octene adsorption at 293 K in a ZSM-5 catalyst by inelastic and quasielastic neutron scattering. J. Phys. Chem. C 123, 417–425 (2019).
Thompson, S. P. et al. Beamline I11 at Diamond: a new instrument for high resolution powder diffraction. Rev. Sci. Instrum. 80, 075107 (2009).
Acknowledgements
We thank EPSRC (EP/P011632/1), the Royal Society and the University of Manchester for funding. We thank the EPSRC National Service for EPR Spectroscopy at the University of Manchester. A.M.S. thanks the Russian Science Foundation (Grant no. 17–73–10320) and Royal Society of Chemistry for funding. We are grateful to Oak Ridge National Laboratory (ORNL), the ISIS Facility and Diamond Light Source (DLS) for access to the beamlines VISION, TOSCA and I11, respectively. We acknowledge DLS for the provision of beamtime at B18 (UK Catalysis Hub SP15151, SP24726) and G. Cibin and V. Celorrio for help at B18 beamline. We acknowledge the support of The University of Manchester’s Dalton Cumbrian Facility (DCF), a partner in the National Nuclear User Facility, the EPSRC UK National Ion Beam Centre and the Henry Royce Institute. We recognize R. Edge and K. Warren for their assistance during the 60Co γ-irradiation processes. We thank A. Jentys from the Technical University of Munich and ISIS Facility for the measurement of the INS spectrum of isobutene as part of RB20053 experimental proposal. We thank C. Webb for help with GC–MS, D. Moulding for help with Raman spectroscopy and M. Kibble for help at the TOSCA beamline. The computing resources were made available through the VirtuES and the ICE-MAN projects, funded by the Laboratory Directed Research and Development programme and by Compute and Data Environment for Science (CADES) at ORNL.
Author information
Authors and Affiliations
Contributions
L.L. and M.F carried out the syntheses and characterization of the zeolite samples. L.L. and X.H. carried out the catalytic tests. L.L., A.M.S., F.T. and E.J.L.M. collected and analysed the EPR data. L.L. and C.M.A.P. collected and analysed the XAS data. L.L., J.H.C., I.D.S. and C.C.T. collected and analysed the synchrotron X-ray diffraction data. Z.T. and Y.L. collected and analysed the Py-IR data. L.L., Y.C., L.L.D., S.R. and A.J.R.-C. collected and analysed the neutron scattering data and carried out the DFT modelling. S.Y. was responsible for the overall direction of the project and preparation of the manuscript, with contributions from all authors.
Corresponding author
Ethics declarations
Competing interests
S.Y. and L.L. are inventors of a patent based on this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes, Figs. 1–24, 1–16 and references.
Crystallographic Data 1
Crystallographic data of NbAlS-1.
Crystallographic Data 2
Crystallographic data of NbAlS-1_GVL.
Crystallographic Data 3
Crystallographic data of NbS-1_GVL.
Crystallographic Data 4
Crystallographic data of ZSM-5_GVL.
Rights and permissions
About this article
Cite this article
Lin, L., Sheveleva, A.M., da Silva, I. et al. Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite. Nat. Mater. 19, 86–93 (2020). https://doi.org/10.1038/s41563-019-0562-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0562-6
This article is cited by
-
Nature-inspired methylated polyhydroxybutyrates from C1 and C4 feedstocks
Nature Chemistry (2023)
-
Construction of C-C bonds via photoreductive coupling of ketones and aldehydes in the metal-organic-framework MFM-300(Cr)
Nature Communications (2021)
-
Investigations of Hydrocarbon Species on Solid Catalysts by Inelastic Neutron Scattering
Topics in Catalysis (2021)
-
Control of zeolite microenvironment for propene synthesis from methanol
Nature Communications (2021)
-
Torn between two sites
Nature Materials (2020)