Abstract
Platinum (Pt)-based materials are important components of microelectronic sensors, anticancer drugs, automotive catalytic converters and electrochemical energy conversion devices1. Pt is currently the most common catalyst used for the oxygen reduction reaction (ORR) in devices such as fuel cells and metal–air batteries2,3, although a scalable use is restricted by the scarcity, cost and vulnerability to poisoning of Pt (refs 4,5,6). Here we show that nanoparticulate zirconium nitride (ZrN) can replace and even surpass Pt as a catalyst for ORR in alkaline environments. As-synthesized ZrN nanoparticles (NPs) exhibit a high oxygen reduction performance with the same activity as that of a widely used Pt-on-carbon (Pt/C) commercial catalyst. Both materials show the same half-wave potential (E1/2 = 0.80 V) and ZrN has a higher stability (ΔE1/2 = −3 mV) than the Pt/C catalyst (ΔE1/2 = −39 mV) after 1,000 ORR cycles in 0.1 M KOH. ZrN is also shown to deliver a greater power density and cyclability than Pt/C in a zinc–air battery. Replacement of Pt by ZrN is likely to reduce costs and promote the usage of electrochemical energy devices, and ZrN may also be useful in other catalytic systems.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding authors upon request.
References
Chen, A. & Holt-Hindle, P. Platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 110, 3767–3804 (2010).
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Li, M. et al. Ultrafine jagged platinum nanowires enable ultrahigh mass activity for the oxygen reduction reaction. Science 354, 1414–1419 (2016).
Cao, B. et al. Cobalt molybdenum oxynitrides: synthesis, structural characterization, and catalytic activity for the oxygen reduction reaction. Angew. Chem. Int. Ed. 52, 10753–10757 (2013).
Chung, H. T. et al. Direct atomic-level insight into the active sites of a high-performance PGM-free ORR catalyst. Science 357, 479–484 (2017).
Wei, W. et al. Nitrogen-doped carbon nanosheets with size-defined mesopores as highly efficient metal-free catalyst for the oxygen reduction reaction. Angew. Chem. Int. Ed. 53, 1570–1574 (2014).
Chu, S., Cui, Y. & Liu, N. The path towards sustainable energy. Nat. Mater. 16, 16–22 (2016).
Liang, Y. et al. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011).
Bashyam, R. & Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 443, 63–66 (2006).
Kumar, A., Ciucci, F., Morozovska, A. N., Kalinin, S. V. & Jesse, S. Measuring oxygen reduction/evolution reactions on the nanoscale. Nat. Chem. 3, 707–713 (2011).
Shen, H. et al. Oxygen reduction reactions of Fe–N–C catalysts: current status and the way forward. Electrochem. Energy Rev. 2, 252–276 (2019).
Zhou, T. et al. A review of radiation-grafted polymer electrolyte membranes for alkaline polymer electrolyte membrane fuel cells. J. Power Sources 293, 946–975 (2015).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
London Metal Exchange (London Metal Exchange); https://www.lme.com/Metals/Precious-metals/Platinum/Averages
Snyder, J., Fujita, T., Chen, M. W. & Erlebacher, J. Oxygen reduction in nanoporous metal–ionic liquid composite electrocatalysts. Nat. Mater. 9, 904–907 (2010).
Hiroyuki, Y., Mikio, K. & Hisao, A. Toyota fuel cell system (TFCS). World Electric Vehicle J. 7, 85–92 (2015).
Zhao, Y. et al. Few-layer graphdiyne doped with sp-hybridized nitrogen atoms at acetylenic sites for oxygen reduction electrocatalysis. Nat. Chem. 10, 934–931 (2018).
D, G. et al. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 16, 361–365 (2016).
Giner, J. & Swette, L. Oxygen reduction on titanium nitride in alkaline electrolyte. Nature 211, 1291–1292 (1966).
Pan, Z. et al. Hollow and porous titanium nitride nanotubes as high-performance catalyst supports for oxygen reduction reaction. J. Mater. Chem. A 2, 13966–13975 (2014).
Cao, M., Di, Z., Cui, Z., Wang, S. & Qin, J. VN hollow spheres assembled from porous nanosheets for high-performance lithium storage and oxygen reduction reaction. J. Mater. Chem. A 4, 7914–7923 (2016).
Youn, D. H. et al. A highly efficient transition metal nitride-based electrocatalyst for oxygen reduction reaction: TiN on a CNT–graphene hybrid support. J. Mater. Chem. A 1, 8007–8015 (2013).
Dong, Y. & Li, J. Tungsten nitride nanocrystals on nitrogen-doped carbon black as efficient electrocatalysts for oxygen reduction reactions. Chem. Commun. 51, 572–575 (2014).
Shanghai Metals Market (Shanghai Metals Market); www.smm.cn/metal/minor-metals
Bo, F. & Lian, G. Synthesis of nanocrystalline zirconium nitride powders by reduction–nitridation of zirconium oxide. J. Am. Ceram. Soc. 87, 696–698 (2010).
Miloiev, I., Strehblow, H. H., GaberLEek, M. & Naviniek, B. Electrochemical oxidation of ZrN hard (PVD) coatings studied by XPS. Surf. Interface. Anal. 24, 448–458 (1996).
Huang, X. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 348, 1230–1234 (2015).
Doi, S., Ishihara, A., Mitsushima, S., Kamiya, N. & Ota, K. Zirconium-based compounds for cathode of polymer electrolyte fuel cell. J. Electrochem. Soc. 154, B362–B369 (2007).
Stamenkovic, V. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 45, 2897–2901 (2006).
Vojvodic, A. & Norskov, J. K. New design paradigm for heterogeneous catalysts. Natl Sci. Rev. 2, 140–149 (2015).
Qiu, Y. & Gao, L. Metal–urea complex—a precursor to metal nitrides. J. Am. Ceram. Soc. 87, 352–357 (2010).
Giordano, C., Erpen, C., Yao, W. & Antonietti, M. Synthesis of Mo and W carbide and nitride nanoparticles via a simple ‘urea glass’ route. Nano Lett. 8, 4659–4663 (2008).
Giordano, C., Erpen, C., Yao, W., Milke, B. & Antonietti, M. Metal nitride and metal carbide nanoparticles by a soft urea pathway. Chem. Mater. 21, 5136–5144 (2009).
Penland, R. B., Mizushima, S., Curran, C. & Quagliano, J. V. Infrared absorption spectra of inorganic coordination complexes. X. Studies of some metal–urea complexes. J. Am. Chem. Soc. 79, 1575–1578 (1957).
Molinari, V., Giordano, C., Antonietti, M. & Esposito, D. Titanium nitride–nickel nanocomposite as heterogeneous catalyst for the hydrogenolysis of aryl ethers. J. Am. Chem. Soc. 136, 1758–1761 (2014).
Chen, X. J. et al. Pressure-induced phonon frequency shifts in transition-metal nitrides. Phys. Rev. B 70, 2199–2208 (2004).
Spengler, W. & Kaiser, R. First and second order Raman scattering in transition metal compounds. Solid State Commun. 18, 881–884 (1976).
Wentzcovitch, P. G. et al. QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 21, 395502 (2009).
Acknowledgements
This work is supported by Natural Science Foundation of China (Grant no. 21471147), National Key Research and Development Plan (Grant no. 2016YFB0101205) and Key Program of the Chinese Academy of Sciences (Grant no. KFZD-SW-320). J.W. thanks the Equipment Research Program (6140721050215) for financial support. M.Y. thanks the National Thousand Youth Talents program of China and Ningbo 3315 program for support. J.P.A. thanks the EPSRC for support.
Author information
Authors and Affiliations
Contributions
Y.Y. and J.W. contributed equally to this work. M.Y. and J.P.A. conceived and coordinated the research. Y.Y. synthesized and characterized the materials, S.A. performed the electronic structure calculations and Y.Y., J.W., H.S., T.T. and R.M. carried out and analysed the electrochemical measurements and co-wrote the paper with M.Y. and J.P.A. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Preparation of ZrN NPs.
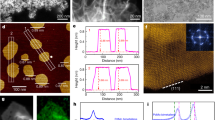
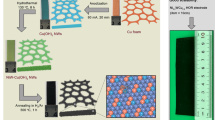
a, Schematic illustration of ZrN NP synthesis. When zirconium chloride was mixed with ethanol, the metal salts dissolved slowly in ethanol to form the corresponding transparent solution. Urea was added into the mixture solution as a nitrogen source. This gives a gel-like amorphous metal-urea precursor that decomposes to ZrN when heated under inert atmosphere. b, FT-IR spectra of pure urea and the zirconium-urea precursor. Urea is bonded to Zr though the formation of oxygen to metal coordinate bonds (C=O→M) in this metal-urea complex, which is evidenced particularly by the decrease of the carbonyl band to 1684 cm−1.
Extended Data Fig. 2 Crystallographic characterization and sample morphologies from SEM.
a, The Rietveld fitted XRD pattern of Zr2ON2 NPs. The reflections are assigned to the cubic Zr2ON2 phase (space group: Ia-3 (206), a = 10.133 Å). b, The Rietveld fitted XRD pattern of ZrO2 NPs. The reflections are indexed by the monoclinic Zr2ON2 phase (space group: P21/c (14), a = 5.145 Å, b = 5.207 Å, c = 5.311 Å, β=99.23º). No additional peaks from impurities are detected in either sample. Their crystal structure are inset. c, SEM image of ZrN NPs. d, Histogram of 200 particle sizes from image c, with a mean size of 45 nm and a standard deviation of 16 nm for the distribution. e, Zr2ON2 NPs. f, ZrO2 NPs. The Zr2ON2 and ZrO2 were designed to have similar sizes to the ZrN NPs for direct comparison of their properties.
Extended Data Fig. 3 Nitrogen adsorption–desorption isotherms of ZrN.
The surface area was determined by the Brunauer-Emmett-Teller (BET) method to be 27.7 m2 g−1.
Extended Data Fig. 4 XPS and Raman spectra.
(a) Wide-range, (b) O 1s region, and (c) N 1s region spectra of ZrN NPs. (d) Wide-range, (e) O 1s region, and (f) N 1s region spectra of Zr2ON2 NPs. (g) Wide-range and (h) O 1s region spectra of ZrO2 NPs. (i) Raman spectrum of ZrN NPs.
Extended Data Fig. 5 Cyclic voltammograms for the catalysts and reproducibility of ORR performance for ZrN NPs.
CV curves of (a) ZrN NPs and (b) Pt/C at a scan rate of 50 mV·s−1 in Ar- and O2-saturated 0.1 M KOH solutions. For ZrN, the quasi-rectangular voltammogram does not show an obvious redox peak in Ar-saturated solution. In contrast, when the electrolyte solution was saturated with O2, a distinct cathodic peak centered at ~0.79 V can be observed (similar to that of Pt/C), indicating pronounced electrocatalytic activity of ZrN NPs for oxygen reduction. c, CV curves of ZrO2, Zr2ON2, and blank glassy carbon disk electrode (GCDE) at a scan rate of 50 mV·s−1 in O2-saturated 0.1 M KOH solution. Both ZrO2 (0.66 V) and Zr2ON2 (0.67 V) samples show more negative cathodic peak potentials compared to ZrN (0.79 V), indicating inferior ORR performance. d, Three independent ORR polarization curves of ZrN NPs in O2-saturated 0.1 KOH electrolyte with a scan rate of 10 mV s−1 at 1600 rpm. The results show that the potential measurements are reproducible to ±10 mV.
Extended Data Fig. 6 ORR catalytic performance data.
RDE polarization curves of (a) Pt/C, (b) ZrN NPs, (c) ZrO2 NPs and (d) Zr2ON2 NPs in O2-saturated 0.1 M KOH at rotation speeds ranging from 400 to 2025 rpm and a scan rate of 10 mV s−1. Koutecky–Levich plots of (e) Pt/C, (f) ZrN NPs, (g) ZrO2 NPs and (h) Zr2ON2 NPs at potentials from 0.4 to 0.6 V. The kinetic current is calculated from the ORR polarization curves by using the Koutecky–Levich equation (1/i =1/ik + 1/id, where ik is the kinetic current, and id is the diffusion-limiting current), which gives linear plots of the inverse of current density (j−1) against the inverse square root of rotation speed (ω−1/2) as shown.
Extended Data Fig. 7 ORR catalytic performances of Pt/C, ZrN NPs, ZrO2 NPs and Zr2ON2 NPs.
a, Linear sweep voltammetry (LSV) curves in O2-saturated 0.1 M KOH solution. ZrN has more positive onset and half-wave potentials than ZrO2 and Zr2ON2, showing that electrocatalytic activity is due to ZrN rather than oxide or oxynitride at the NP surface. b, Ring current densities. c, H2O2 yields calculated from the ring and disk currents. The calculated H2O2 yield is below ~10 % for ZrN at all investigated potentials. d, Electron transfer numbers, which are directly related to the H2O2 yields. e, The RDE polarization curves of ZrN NPs before and after cycles with a rotation speed of 1600 rpm and scan rate of 10 mV s−1. The ADTs of the catalysts were carried out by cycling the potential between 0.6 V and 1.2 V in a 0.1 M KOH solution exposed to air at room temperature for evaluation of the long-term electrochemical durability of the catalysts. After 8000 cycles the half-wave potential E1/2 exhibits only a small negative shift of 7 mV, evidencing the high durability of the ZrN NPs. f, Chronoamperometric response of ZrN NPs at a constant potential in O2-saturated 6 M KOH solution. ZrN NPs exhibit high durability under concentrated alkaline condition and retain a relative current of 87.9 % after 18 h.
Extended Data Fig. 8 ORR catalytic performances of ZrN NPs on a GDE.
a, CV curves of ZrN NPs on a GDE or a glassy carbon disk electrode (GCDE) at a scan rate of 50 mV·s−1 in Ar- and O2-saturated 0.1 M KOH solutions. The GDE oxygen reduction peak at 0.81 V in the O2-saturated electrolyte is at a slightly higher potential than that of ZrN NPs on a GCDE. b, LSV curves of the ZrN NPs and Pt/C on a GDE or a GCDE at a scan rate of 10 mV·s−1 in O2-saturated 0.1 M KOH solution. Both the onset and half-wave potentials of ZrN NPs on the GDE are close to those for the GCDE, although the GDE has a higher limited diffusion current due to the difference between electrical conductivities of the two electrodes. c, RDE polarization curves of ZrN NPs on the GDE in O2-saturated 0.1 M KOH at rotation speeds ranging from 400 to 2025 rpm and a scan rate of 10 mV·s−1. d, Koutecky–Levich plots of ZrN NPs on the GDE at potentials from 0.4 to 0.6 V. On the basis of the slopes of the Koutecky-Levich plots, the number of transferred electrons is 4, which is same as that obtained using the GCDE. These results demonstrate that C from the glassy carbon electrode is not required for catalytic activity, indicating that the activity of ZrN is intrinsic.
Extended Data Fig. 9 Zinc-air battery with catalysts as the cathode.
a, Schematic for fabrication of the zinc–air battery. The electrolyte used was 6 M KOH with 0.2 M zinc acetate and a polished Zn foil was used as the anode. The air-cathodes for Zn-air battery were prepared by coating the catalysts on a hydrophobic carbon paper. b, Photograph of the zinc-air battery.
Extended Data Fig. 10 Calculated electronic densities of states.
(a) Pt and (b) ZrN. Both materials are metallic with finite DOS at the (zero) Fermi energy.
Supplementary information
Supplementary Information
Supplementary Methods 1 and 2 and Tables 1 and 2.
Rights and permissions
About this article
Cite this article
Yuan, Y., Wang, J., Adimi, S. et al. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 19, 282–286 (2020). https://doi.org/10.1038/s41563-019-0535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0535-9
This article is cited by
-
In situ modulating coordination fields of single-atom cobalt catalyst for enhanced oxygen reduction reaction
Nature Communications (2024)
-
Highly Active Mesoporous Zirconium Nitride Immobilized on SiO2 Synthesized by Complex-Assisted Method with EDTA and KHP for Catalytic Hydroconversion of Crude Palm Oil
Catalysis Surveys from Asia (2024)
-
Unlocking single-atom catalysts via amorphous substrates
Nano Research (2024)
-
Hydroconversion of Crude Palm Oil Over Highly Dispersed Porous Silica Modified Zirconium Nitride: Effect of EDTA and KHF Template
Silicon (2024)
-
Improved Catalytic Properties of Fluorine-Doped La0.6Sr0.4Co0.2Fe0.8O3-δ for Air Electrode with High-Performance Metal-Air Batteries
Electronic Materials Letters (2024)