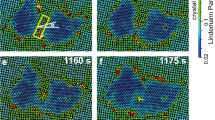
Abstract
Nucleation and growth are universally important in systems from the atomic to the micrometre scale as they dictate structural and functional attributes of crystals. However, at the nanoscale, the pathways towards crystallization have been largely unexplored owing to the challenge of resolving the motion of individual building blocks in a liquid medium. Here we address this gap by directly imaging the full transition of dispersed gold nanoprisms to a superlattice at the single-particle level. We utilize liquid-phase transmission electron microscopy at low dose rates to control nanoparticle interactions without affecting their motions. Combining particle tracking with Monte Carlo simulations, we reveal that positional ordering of the superlattice emerges from orientational disorder. This method allows us to measure parameters such as line tension and phase coordinates, charting the nonclassical nucleation pathway involving a dense, amorphous intermediate. We demonstrate the versatility of our approach via crystallization of different nanoparticles, pointing the way to more general applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
Code availability
Custom Matlab codes for image processing and particle tracking as well as the algorithms for the particle interactions and the MC simulations are available from the corresponding authors upon request.
References
De Yoreo, J. J. et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349, aaa6760 (2015).
Loh, N. D. et al. Multistep nucleation of nanocrystals in aqueous solution. Nat. Chem. 9, 77–82 (2017).
Li, B., Zhou, D. & Han, Y. Assembly and phase transitions of colloidal crystals. Nat. Rev. Mater. 1, 15011 (2016).
Henzler, K. et al. Supersaturated calcium carbonate solutions are classical. Sci. Adv. 4, eaao6283 (2018).
Chen, J., Sarma, B., Evans, J. M. B. & Myerson, A. S. Pharmaceutical crystallization. Cryst. Growth Des. 11, 887–895 (2011).
John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 58, 2486–2489 (1987).
Choi, J.-H. et al. Exploiting the colloidal nanocrystal library to construct electronic devices. Science 352, 205–208 (2016).
Noorduin, W. L., Grinthal, A., Mahadevan, L. & Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837 (2013).
Ten Wolde, P. R. & Frenkel, D. Enhancement of protein crystal nucleation by critical density fluctuations. Science 277, 1975–1978 (1997).
Ducrot, É., He, M., Yi, G.-R. & Pine, D. J. Colloidal alloys with preassembled clusters and spheres. Nat. Mater. 16, 652–657 (2017).
Zhang, T. H. & Liu, X. Y. How does a transient amorphous precursor template crystallization. J. Am. Chem. Soc. 129, 13520–13526 (2007).
Tan, P., Xu, N. & Xu, L. Visualizing kinetic pathways of homogeneous nucleation in colloidal crystallization. Nat. Phys. 10, 73–79 (2013).
Kenneth, G. L. The physics of snow crystals. Rep. Prog. Phys. 68, 855–895 (2005).
Ye, X. et al. Quasicrystalline nanocrystal superlattice with partial matching rules. Nat. Mater. 16, 214–219 (2017).
Xia, Y. et al. Self-assembly of self-limiting monodisperse supraparticles from polydisperse nanoparticles. Nat. Nanotechnol. 6, 580–587 (2011).
Lin, H. et al. Clathrate colloidal crystals. Science 355, 931–935 (2017).
Batista, C. A., Larson, R. G. & Kotov, N. A. Nonadditivity of nanoparticle interactions. Science 350, 1242477 (2015).
De Yoreo, J. J. & Sommerdijk, N. A. J. M. Investigating materials formation with liquid-phase and cryogenic TEM. Nat. Rev. Mater. 1, 16035 (2016).
Park, J. et al. 3D structure of individual nanocrystals in solution by electron microscopy. Science 349, 290–295 (2015).
Liao, H.-G., Cui, L., Whitelam, S. & Zheng, H. Real-time imaging of Pt3Fe nanorod growth in solution. Science 336, 1011–1014 (2012).
Sutter, E. et al. In situ microscopy of the self-assembly of branched nanocrystals in solution. Nat. Commun. 7, 11213 (2016).
Schneider, N. M. et al. Electron–water interactions and implications for liquid cell electron microscopy. J. Phys. Chem. C. 118, 22373–22382 (2014).
Kim, J., Ou, Z., Jones, M. R., Song, X. & Chen, Q. Imaging the polymerization of multivalent nanoparticles in solution. Nat. Commun. 8, 761 (2017).
Agarwal, U. & Escobedo, F. A. Mesophase behaviour of polyhedral particles. Nat. Mater. 10, 230–235 (2011).
Jones, M. R. et al. DNA-nanoparticle superlattices formed from anisotropic building blocks. Nat. Mater. 9, 913–917 (2010).
Fraser, D. P., Zuckermann, M. J. & Mouritsen, O. G. Simulation technique for hard-disk models in two dimensions. Phys. Rev. A 42, 3186–3195 (1990).
Savage, J. R., Blair, D. W., Levine, A. J., Guyer, R. A. & Dinsmore, A. D. Imaging the sublimation dynamics of colloidal crystallites. Science 314, 795–798 (2006).
Liu, W. et al. Diamond family of nanoparticle superlattices. Science 351, 582–586 (2016).
Sauter, A. et al. On the question of two-step nucleation in protein crystallization. Faraday Discuss. 179, 41–58 (2015).
Lutsko, J. F. & Nicolis, G. Theoretical evidence for a dense fluid precursor to crystallization. Phys. Rev. Lett. 96, 046102 (2006).
Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 409, 1020–1023 (2001).
Donev, A., Burton, J., Stillinger, F. H. & Torquato, S. Tetratic order in the phase behavior of a hard-rectangle system. Phys. Rev. B 73, 054109 (2006).
Timmermans, J. Plastic crystals: a historical review. J. Phys. Chem. Solids 18, 1–8 (1961).
Liu, B. et al. Switching plastic crystals of colloidal rods with electric fields. Nat. Commun. 5, 3092 (2014).
Boles, M. A., Engel, M. & Talapin, D. V. Self-assembly of colloidal nanocrystals: from intricate structures to functional materials. Chem. Rev. 116, 11220–11289 (2016).
Gruebele, M., Dave, K. & Sukenik, S. Globular protein folding in vitro and in vivo. Annu. Rev. Biophys. 45, 233–251 (2016).
Nikoobakht, B. & El-Sayed, M. A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003).
O’Brien, M. N., Jones, M. R., Brown, K. A. & Mirkin, C. A. Universal noble metal nanoparticle seeds realized through iterative reductive growth and oxidative dissolution reactions. J. Am. Chem. Soc. 136, 7603–7606 (2014).
Acknowledgements
The experiments and analysis of the experimental data presented in this research were supported by the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering under award no. DE-FG02-07ER46471 through the Materials Research Laboratory at the University of Illinois (the nanoprism system) and the National Science Foundation through award no. DMR-1752517 (the concave nanocube and nanosphere systems). The theoretical model and simulations presented in this research were supported by the National Science Foundation through award no. DMR-1610796 and based upon work supported as part of the Center for Bio-Inspired Energy Science, an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under Award DE-SC0000989. Z.W. gratefully acknowledges support from a Ryan Fellowship and the International Institute for Nanotechnology at Northwestern University. We thank J. Kim for useful discussions.
Author information
Authors and Affiliations
Contributions
Z.O. and Q.C. designed and performed the experiments and analysed the data on the nanoprism system. Z.W. and E.L. designed the theoretical model and performed the simulations. B.L. performed the experiments on the concave nanocube and nanosphere systems. Z.O., Z.W., E.L. and Q.C. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Video Legends 1–9, Notes 1–13, Figures 1–28, Tables 1–4 and references.
Supplementary Video 1
Synchronized liquid-phase TEM video showing the lattice vibration inside a large-scale hexagonal superlattice formed by triangular nanoprisms.
Supplementary Video 2
Liquid-phase TEM video of individual prisms moving on the SiNx substrate and the stacking process of a pair of prisms.
Supplementary Video 3
Monte Carlo simulation of hexagonal superlattice formation from 1472 triangular prisms.
Supplementary Video 4
Liquid-phase TEM video showing the disassembly and reassembly of the lattice when the electron beam is turned off or on.
Supplementary Video 5
Single-column Monte Carlo simulation showing the fluctuation of prism orientations inside a column.
Supplementary Video 6
Synchronized liquid-phase TEM video showing the formation of a large-scale superlattice.
Supplementary Video 7
Monte Carlo simulation of the formation of side-by-side aggregates from 576 triangular prisms.
Supplementary Video 8
Liquid-phase TEM video showing the crystallization of gold concave nanocubes into simple cubic superlattices.
Supplementary Video 9
Liquid-phase TEM video showing the crystallization of nanospheres into face-centred cubic superlattices.
Rights and permissions
About this article
Cite this article
Ou, Z., Wang, Z., Luo, B. et al. Kinetic pathways of crystallization at the nanoscale. Nat. Mater. 19, 450–455 (2020). https://doi.org/10.1038/s41563-019-0514-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0514-1
This article is cited by
-
Non-classical crystallization in soft and organic materials
Nature Reviews Materials (2024)
-
Unravelling crystal growth of nanoparticles
Nature Nanotechnology (2023)
-
Atomic-scale observation of non-classical nucleation-mediated phase transformation in a titanium alloy
Nature Materials (2022)
-
Observing short-range orientational order in small-molecule liquids
Scientific Reports (2022)
-
Enantiomer-dependent immunological response to chiral nanoparticles
Nature (2022)