Abstract
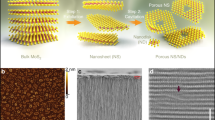
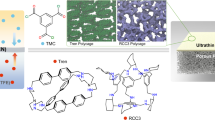
Nanolaminate membranes made of two-dimensional materials such as graphene oxide are promising candidates for molecular sieving via size-limited diffusion in the two-dimensional capillaries, but high hydrophilicity makes these membranes unstable in water. Here, we report a nanolaminate membrane based on covalently functionalized molybdenum disulfide (MoS2) nanosheets. The functionalized MoS2 membranes demonstrate >90% and ~87% rejection for micropollutants and NaCl, respectively, when operating under reverse osmotic conditions. The sieving performance and water flux of the functionalized MoS2 membranes are attributed both to control of the capillary widths of the nanolaminates and to control of the surface chemistry of the nanosheets. We identify small hydrophobic functional groups, such as the methyl group, as the most promising for water purification. Methyl- functionalized nanosheets show high water permeation rates as confirmed by our molecular dynamic simulations, while maintaining high NaCl rejection. Control of the surface chemistry and the interlayer spacing therefore offers opportunities to tune the selectivity of the membranes while enhancing their stability.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Abraham, J. et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 12, 546–550 (2017).
Kim, H. W. et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 342, 91–95 (2013).
Joshi, R. K. et al. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 343, 752–754 (2014).
Chen, L. et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 550, 380–383 (2017).
Han, Y., Xu, Z. & Gao, C. Ultrathin graphene nanofiltration membrane for water purification. Adv. Funct. Mater. 23, 3693–3700 (2013).
Hirunpinyopas, W. et al. Desalination and nanofiltration through functionalized laminar MoS2 membranes. ACS Nano 11, 11082–11090 (2017).
Heiranian, M., Farimani, A. B. & Aluru, N. R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 6, 8616 (2015).
Nair, R. R., Wu, H. A., Jayaram, P. N., Grigorieva, I. V. & Geim, A. K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 335, 442–444 (2012).
Koltonow, A. R. & Huang, J. Two-dimensional nanofluidics. Science 351, 1395–1396 (2016).
Wei, N., Peng, X. & Xu, Z. Understanding water permeation in graphene oxide membranes. ACS Appl. Mater. Interfaces 6, 5877–5883 (2014).
Wei, N., Peng, X. & Xu, Z. Breakdown of fast water transport in graphene oxides. Phys. Rev. E 89, 012113 (2014).
Sun, L., Huang, H. & Peng, X. Laminar MoS2 membranes for molecule separation. Chem. Commun. 49, 10718–10720 (2013).
Sun, L. et al. Ultrafast molecule separation through layered WS2 nanosheet membranes. ACS Nano 8, 6304–6311 (2014).
Wang, Z. et al. Understanding the aqueous stability and filtration capability of MoS2 membranes. Nano Lett. 17, 7289–7298 (2017).
Deng, M., Kwac, K., Li, M., Jung, Y. & Park, H. G. Stability, molecular sieving, and ion diffusion selectivity of a lamellar membrane from two-dimensional molybdenum disulfide. Nano Lett. 17, 2342–2348 (2017).
Achari, A., Sahana, S. & Eswaramoorthy, M. High performance MoS2 membranes: effects of thermally driven phase transition on CO2 separation efficiency. Energy Environ. Sci. 9, 1224–1228 (2016).
Joensen, P., Frindt, R. F. & Morrison, S. R. Single-layer MoS2. Mater. Res. Bull. 21, 457–461 (1986).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Voiry, D. et al. Covalent functionalization of monolayered transition metal dichalcogenides by phase engineering. Nat. Chem. 7, 45–49 (2015).
Wang, H. et al. MoSe2 and WSe2 nanofilms with vertically aligned molecular layers on curved and rough surfaces. Nano Lett. 13, 3426–3433 (2013).
Eda, G., Fanchini, G. & Chhowalla, M. Large-area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 3, 270–274 (2008).
Acerce, M., Voiry, D. & Chhowalla, M. Metallic 1T phase MoS2 nanosheets as supercapacitor electrode materials. Nat. Nanotechnol. 10, 313–318 (2015).
Yeh, C.-N., Raidongia, K., Shao, J., Yang, Q.-H. & Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat Chem 7, 166–170 (2015).
Holt, J. K. et al. Fast mass transport through Sub-2-Nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Secchi, E. et al. Massive radius-dependent flow slippage in carbon nanotubes. Nature 537, 210–213 (2016).
Balme, S. et al. Unexpected ionic transport behavior on hydrophobic and uncharged conical nanopore. Faraday Discuss. 210, 69–85 (2018).
Balme, S. et al. Ionic transport through sub-10 nm diameter hydrophobic high-aspect ratio nanopores: experiment, theory and simulation. Sci. Rep. 5, 10135 (2015).
Morelos-Gomez, A. et al. Effective NaCl and dye rejection of hybrid graphene oxide/graphene layered membranes. Nat. Nanotechnol. 12, 1083–1088 (2017).
Han, Y., Jiang, Y. & Gao, C. High-Flux graphene oxide nanofiltration membrane intercalated by carbon nanotubes. ACS Appl. Mater. Interfaces 7, 8147–8155 (2015).
Zhao, G., Hu, R., Zhao, X., He, Y. & Zhu, H. High flux nanofiltration membranes prepared with a graphene oxide homo-structure. J. Membr. Sci. 585, 29–37 (2019).
van Duin, A. C. T., Dasgupta, S., Lorant, F. & Goddard, W. A. ReaxFF: a reactive force field for hydrocarbons. J. Phys. Chem. A 105, 9396–9409 (2001).
Lupkowski, M. & van Swol, F. Computer simulation of fluids interacting with fluctuating walls. J. Chem. Phys. 93, 737–745 (1990).
Kwac, K. et al. Multilayer Two-Dimensional water structure confined in MoS2. J. Phys. Chem. C 121, 16021–16028 (2017).
Chandra, A. Effects of ion atmosphere on hydrogen-bond dynamics in aqueous electrolyte solutions. Phys. Rev. Lett. 85, 768 (2000).
Kappera, R. et al. Phase-engineered low-resistance contacts for ultrathin MoS2 transistors. Nat. Mater. 13, 1128–1134 (2014).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Kim, S.-Y. & van Duin, A. C. T. Simulation of titanium metal/titanium dioxide etching with chlorine and hydrogen chloride gases using the ReaxFF reactive force field. J. Phys. Chem. A 117, 5655–5663 (2013).
Ostadhossein, A. et al. ReaxFF reactive force-field study of molybdenum disulfide (MoS2). J. Phys. Chem. Lett. 8, 631–640 (2017).
Plimpton, S. Fast Parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Acknowledgements
L.R. acknowledges scholarship from the Graduate School ‘Ecole doctorale des Sciences Chimiques Balard, ED 459’. D.V. acknowledges financial supports from ‘Project Axe Transverse Santé’ and CNRS Cellule Energie exploratory project ‘NANOSMO’. This project has also received partial funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant no. 804320). The French Région Ile de France – SESAME programme is acknowledged for financial support (700 MHz NMR spectrometer). We thank The Hong Kong Polytechnic University and the Department of Applied Physics for the computational resources. D. Cot and E. Oliviero are acknowledged for support with the electron microscopy. We thank V. Flaud and L. Causse for the X-ray photoelectron spectroscopy and the inductively coupled plasma optical emission spectrometry measurements.
Author information
Authors and Affiliations
Contributions
D.V. conceived the idea, designed the experiments and wrote the manuscript. L.R. designed the experiments with D.V., fabricated the membranes and performed membrane characterizations and analysed the results. L.R. and D.V. analysed the data and wrote the manuscript. E.P. carried out high-performance liquid chromatography and liquid NMR spectroscopy measurements. T.M. performed Raman spectroscopy measurements with L.R. and discussed the results with D.V. and L.R.. C.C.D. and C.G. performed 13C CAS NMR spectroscopy measurements and C.S. discussed the results with D.V. and L.R.. S.B. and M.B. assisted L.R. on ionic permeation experiments and discussed the water permeation results. N.O. performed the MD simulations and wrote the manuscript with D.V. and L.R. P.M. discussed the results with D.V. and L.R. All of the authors edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary materials and methods, Supplementary Figs. 1–43, Supplementary Tables 1–10 and Supplementary refs. 1–57
Rights and permissions
About this article
Cite this article
Ries, L., Petit, E., Michel, T. et al. Enhanced sieving from exfoliated MoS2 membranes via covalent functionalization. Nat. Mater. 18, 1112–1117 (2019). https://doi.org/10.1038/s41563-019-0464-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0464-7
This article is cited by
-
Nanowire-assisted electrochemical perforation of graphene oxide nanosheets for molecular separation
Nature Communications (2024)
-
Recent advances on liquid intercalation and exfoliation of transition metal dichalcogenides: From fundamentals to applications
Nano Research (2024)
-
2D nanochannels and huge specific surface area offer unique ways for water remediation and adsorption: assessing the strengths of hexagonal boron nitride in separation technology
Functional Composite Materials (2023)
-
Towards the realisation of high permi-selective MoS2 membrane for water desalination
npj Clean Water (2023)
-
High-surface-area functionalized nanolaminated membranes for energy-efficient nanofiltration and desalination in forward osmosis
Nature Water (2023)