Abstract
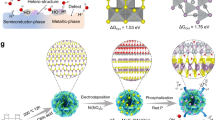
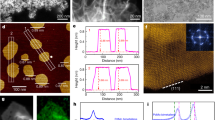
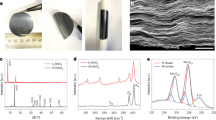
Metallic transition metal dichalcogenides (TMDs)1,2,3,4,5,6,7,8 are good catalysts for the hydrogen evolution reaction (HER). The overpotential and Tafel slope values of metallic phases and edges9 of two-dimensional (2D) TMDs approach those of Pt. However, the overall current density of 2D TMD catalysts remains orders of magnitude lower (~10–100 mA cm−2) than industrial Pt and Ir electrolysers (>1,000 mA cm−2)10,11. Here, we report the synthesis of the metallic 2H phase of niobium disulfide with additional niobium (2H Nb1+xS2, where x is ~0.35)12 as a HER catalyst with current densities of >5,000 mA cm−2 at ~420 mV versus a reversible hydrogen electrode. We find the exchange current density at 0 V for 2H Nb1.35S2 to be ~0.8 mA cm−2, corresponding to a turnover frequency of ~0.2 s−1. We demonstrate an electrolyser based on a 2H Nb1+xS2 cathode that can generate current densities of 1,000 mA cm−2. Our theoretical results reveal that 2H Nb1+xS2 with Nb-terminated surface has free energy for hydrogen adsorption that is close to thermoneutral, facilitating HER. Therefore, 2H Nb1+xS2 could be a viable catalyst for practical electrolysers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
References
Lukowski, M. A. et al. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 135, 10274–10277 (2013).
Voiry, D. et al. Conducting MoS2 nanosheets as catalysts for hydrogen evolution reaction. Nano Lett. 13, 6222–6227 (2013).
Voiry, D. et al. Enhanced catalytic activity in strained chemically exfoliated WS2 nanosheets for hydrogen evolution. Nat. Mater. 12, 850 (2013).
Liu, Y. et al. Self-optimizing, highly surface-active layered metal dichalcogenide catalysts for hydrogen evolution. Nat. Energy 2, 17127 (2017).
Shi, J. et al. Two-dimensional metallic tantalum disulfide as a hydrogen evolution catalyst. Nat. Commun. 8, 958 (2017).
Li, H. et al. Atomic-sized pores enhanced electrocatalysis of TaS2 nanosheets for hydrogen evolution. Adv. Mater. 28, 8945–8949 (2016).
Yuan, J. et al. Facile synthesis of single crystal vanadium disulfide nanosheets by chemical vapor deposition for efficient hydrogen evolution reaction. Adv. Mater. 27, 5605–5609 (2015).
Chia, X., Ambrosi, A., Lazar, P., Sofer, Z. & Pumera, M. Electrocatalysis of layered Group 5 metallic transition metal dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 4, 14241–14253 (2016).
Jaramillo, T. F. et al. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 317, 100–102 (2007).
Zhigang, S., Baolian, Y. & Ming, H. Bifunctional electrodes with a thin catalyst layer for ‘unitized’ proton exchange membrane regenerative fuel cell. J. Power Sources 79, 82–85 (1999).
Altmann, S., Kaz, T. & Friedrich, K. A. Bifunctional electrodes for unitised regenerative fuel cells. Electrochim. Acta 56, 4287–4293 (2011).
Jellinek, F., Brauer, G. & Müller, H. Molybdenum and niobium sulphides. Nature 185, 376 (1960).
Merki, D. & Hu, X. Recent developments of molybdenum and tungsten sulfides as hydrogen evolution catalysts. Energy Environ. Sci. 4, 3878–3888 (2011).
Benck, J. D., Hellstern, T. R., Kibsgaard, J., Chakthranont, P. & Jaramillo, T. F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 4, 3957–3971 (2014).
Voiry, D. et al. The role of electronic coupling between substrate and 2D MoS2 nanosheets in electrocatalytic production of hydrogen. Nat. Mater. 15, 1003 (2016).
Yin, Y. et al. Contributions of phase, sulfur vacancies, and edges to the hydrogen evolution reaction catalytic activity of porous molybdenum disulfide nanosheets. J. Am. Chem. Soc. 138, 7965–7972 (2016).
Kibsgaard, J., Chen, Z., Reinecke, B. N. & Jaramillo, T. F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 11, 963 (2012).
Kong, D. et al. Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett. 13, 1341–1347 (2013).
Tsai, C., Abild-Pedersen, F. & Nørskov, J. K. Tuning the MoS2 edge-site activity for hydrogen evolution via support interactions. Nano Lett. 14, 1381–1387 (2014).
Eda, G. et al. Photoluminescence from chemically exfoliated MoS2. Nano Lett. 11, 5111–5116 (2011).
Pan, H. Metal dichalcogenides monolayers: novel catalysts for electrochemical hydrogen production. Sci. Rep. 4, 5348 (2014).
Tsai, C., Chan, K., Nørskov, J. K. & Abild-Pedersen, F. Theoretical insights into the hydrogen evolution activity of layered transition metal dichalcogenides. Surf. Sci. 640, 133–140 (2015).
Yu, Y. et al. Layer-dependent electrocatalysis of MoS2 for hydrogen evolution. Nano Lett. 14, 553–558 (2014).
Han, N. et al. Nitrogen-doped tungsten carbide nanoarray as an efficient bifunctional electrocatalyst for water splitting in acid. Nat. Commun. 9, 924 (2018).
Kibsgaard, J., Jaramillo, T. F. & Besenbacher, F. Building an appropriate active-site motif into a hydrogen-evolution catalyst with thiomolybdate [Mo3S13]2− clusters. Nat. Chem. 6, 248 (2014).
Xie, J. et al. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 25, 5807–5813 (2013).
Hellstern, T. R., Benck, J. D., Kibsgaard, J., Hahn, C. & Jaramillo, T. F. Engineering cobalt phosphide (CoP) thin film catalysts for enhanced hydrogen evolution activity on silicon photocathodes. Adv. Energy Mater. 6, 1501758 (2016).
Wu, T. et al. Crystallographic facet dependence of the hydrogen evolution reaction on CoPS: theory and experiments. ACS Catal. 8, 1143–1152 (2018).
Li, H. et al. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nat. Mater. 15, 48 (2016).
Li, S. et al. Halide-assisted atmospheric pressure growth of large WSe2 and WS2 monolayer crystals. Appl. Mater. Today 1, 60–66 (2015).
Suh, J. et al. Doping against the native propensity of MoS2: degenerate hole doping by cation substitution. Nano Lett. 14, 6976–6982 (2014).
Huang, Y. H., Peng, C. C., Chen, R. S., Huang, Y. S. & Ho, C. H. Transport properties in semiconducting NbS2 nanoflakes. Appl. Phys. Lett. 105, 93106 (2014).
Molenda, J., Bak, T. & Marzec, J. Electrical and electrochemical properties of niobium disulphide. Phys. Status Solidi A 156, 159–168 (1996).
Niazi, A. & Rastogi, A. K. Low-temperature resistance minimum in non-superconducting 3R-Nb1+xS2 and 3R-GaxNbS2. J. Phys. Condens. Matter 13, 6787 (2001).
Zhao, S. et al. Two-dimensional metallic NbS: growth, optical identification and transport properties. 2D Mater. 3, 25027 (2016).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Klimeš, J., Bowler, D. R. & Michaelides, A. Chemical accuracy for the van der Waals density functional. J. Phys. Condens. Matter 22, 22201 (2010).
Wellendorff, J. et al. Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys. Rev. B 85, 235149 (2012).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Acknowledgements
M.C. and J.Y. acknowledge financial support from AFOSR grant no. FA9550-16-1-0289. M.C. and Y.W. acknowledge support from NSF grant no. ECCS-1608389. M.C., W.Z. and X.S. acknowledge support from Shenzhen Peacock Plan (grant no. KQTD2016053112042971). M.C. and A.R.M. acknowledge financial support from the Ministry of Higher Education Malaysia. H.Y.J. acknowledges support from Creative Materials Discovery Programme through the National Research Foundation of Korea (grant no. NRF-2016M3D1A1900035). E.J.G.S. acknowledges the use of computational resources from the UK National High-performance Computing Service (ARCHER) for which access was obtained via the UKCP consortium (EPSRC grant no. EP/K013564/1), and the UK Materials and Molecular Modelling Hub for access to the THOMAS supercluster, which is partially funded by EPSRC (grant no. EP/P020194/1). The Queen’s Fellow Award through grant no. M8407MPH, the Enabling Fund (grant no. A5047TSL) and the Department for the Economy (grant no. USI 097) are also acknowledged by E.J.G.S.
Author information
Authors and Affiliations
Contributions
M.C. conceived the idea and supervised the project. J.Y. and A.R.M. designed the experiments with guidance from M.C. J.Y. performed the electrochemical measurements and analyses with advice from R.F. and D.V. A.R.M. synthesized the Nb1.35S2 samples and characterized them. Y.W. made the devices for the HER measurements and took the electrical measurements. X.S. and I.B. made the NbS2 samples and characterized them with the help of F.Z. and W.Z. H.Y.J. prepared the focused ion beam samples and performed the STEM analyses on the samples. M.A. and E.J.G.S. provided theoretical insight for the experimental results. M.C., H.Y.J., J.Y., D.V., R.F. and H.S.S. analysed the data. M.C. wrote the paper with J.Y. and all of the authors edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13 and Supplementary Tables 1–8
Rights and permissions
About this article
Cite this article
Yang, J., Mohmad, A.R., Wang, Y. et al. Ultrahigh-current-density niobium disulfide catalysts for hydrogen evolution. Nat. Mater. 18, 1309–1314 (2019). https://doi.org/10.1038/s41563-019-0463-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0463-8
This article is cited by
-
Direct visualization of stacking-selective self-intercalation in epitaxial Nb1+xSe2 films
Nature Communications (2024)
-
From VIB- to VB-Group Transition Metal Disulfides: Structure Engineering Modulation for Superior Electromagnetic Wave Absorption
Nano-Micro Letters (2024)
-
Key role of electron accessibility at the noble metal-free catalytic interface in hydrogen evolution reaction
Nano Research (2024)
-
Anchoring Ru clusters to highly defective N-doped carbon nanotubes via a thermal-shock strategy for stable industrial hydrogen evolution
Nano Research (2024)
-
Unraveling and leveraging in situ surface amorphization for enhanced hydrogen evolution reaction in alkaline media
Nature Communications (2023)