Abstract
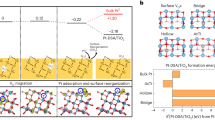
The use of oxide-supported isolated Pt-group metal atoms as catalytic active sites is of interest due to their unique reactivity and efficient metal utilization. However, relationships between the structure of these active sites, their dynamic response to environments and catalytic functionality have proved difficult to experimentally establish. Here, sinter-resistant catalysts where Pt was deposited uniformly as isolated atoms in well-defined locations on anatase TiO2 nanoparticle supports were used to develop such relationships. Through a combination of in situ atomic-resolution microscopy- and spectroscopy-based characterization supported by first-principles calculations it was demonstrated that isolated Pt species can adopt a range of local coordination environments and oxidation states, which evolve in response to varied environmental conditions. The variation in local coordination showed a strong influence on the chemical reactivity and could be exploited to control the catalytic performance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data reported in this paper are available from the corresponding author upon request.
References
Campbell, C. T., Parker, S. C. & Starr, D. E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 298, 811–814 (2002).
Fu, Q., Saltsburg, H. & Flytzani-Stephanopoulos, M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts. Science 301, 935–938 (2003).
Van’t Blik, H. F. J. et al. Structure of rhodium in an ultradispersed Rh/Al2O3 catalyst as studied by EXAFS and other techniques. J. Am. Chem. Soc. 107, 3139–3147 (1985).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 353, 150–154 (2016).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Kistler, J. D. et al. A single-site platinum CO oxidation catalyst in zeolite KLTL: microscopic and spectroscopic determination of the locations of the platinum atoms. Angew. Chem. 126, 9050–9053 (2014).
Matsubu, J. C., Yang, V. N. & Christopher, P. Isolated metal active site concentration and stability controls catalytic CO2 reduction selectivity. J. Am. Chem. Soc. 137, 3076–3084 (2015).
Flytzani-Stephanopoulos, M. & Gates, B. C. Atomically dispersed supported metal catalysts. Annu. Rev. Chem. Biomol. Eng. 3, 545–574 (2012).
Bruix, A. et al. Maximum noble-metal efficiency in catalytic materials: atomically dispersed surface platinum. Angew. Chem. Int. Ed. 53, 10525–10530 (2014).
Hoffman, A. S., Fang, C.-Y. & Gates, B. C. Homogeneity of surface sites in supported single-site metal catalysts: assessment with band widths of metal carbonyl infrared spectra. J. Phys. Chem. Lett. 7, 3854–3860 (2016).
Parkinson, G. S. et al. Carbon monoxide-induced adatom sintering in a Pd–Fe3O4 model catalyst. Nat. Mater. 12, 724–728 (2013).
Hoffman, A. S. et al. Beating heterogeneity of single-site catalysts: MgO-supported iridium complexes. ACS Catal. 8, 3489–3498 (2018).
Tauster, S. J., Fung, S. C. & Garten, R. L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 100, 170–175 (1978).
Gänzler, A. M. et al. Tuning the structure of platinum particles on ceria in situ for enhancing the catalytic performance of exhaust gas catalysts. Angew. Chemie Int. Ed. 56, 13078–13082 (2017).
Zugic, B. et al. Dynamic restructuring drives catalytic activity on nanoporous gold–silver alloy catalysts. Nat. Mater. 16, 558–564 (2016).
Liu, L. et al. Generation of subnanometric platinum with high stability during transformation of a 2D zeolite into 3D. Nat. Mater. 16, 132–138 (2017).
Moliner, M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. J. Am. Chem. Soc. 138, 15743–15750 (2016).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Schreier, M. & Regalbuto, J. R. A fundamental study of Pt tetraammine impregnation of silica: 1. The electrostatic nature of platinum adsorption. J. Catal. 225, 190–202 (2004).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters, and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Kale, M. J. & Christopher, P. Utilizing quantitative in situ FTIR spectroscopy to identify well-coordinated Pt atoms as the active site for CO oxidation on Al2O3-supported Pt catalysts. ACS Catal. 6, 5599–5609 (2016).
Avanesian, T. et al. Quantitative and atomic-scale view of CO-induced Pt nanoparticle surface reconstruction at saturation coverage via DFT calculations coupled with in situ TEM and IR. J. Am. Chem. Soc. 139, 4551–4558 (2017).
Therrien, A. J. et al. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 1, 192–198 (2018).
Ivanova, E., Mihaylov, M., Thibault-Starzyk, F., Daturi, M. & Hadjiivanov, K. FTIR spectroscopy study of CO and NO adsorption and co-adsorption on Pt/TiO2. J. Mol. Catal. A 274, 179–184 (2007).
Aleksandrov, H. A., Neyman, K. M., Hadjiivanov, K. I. & Vayssilov, G. N. Can the state of platinum species be unambiguously determined by the stretching frequency of an adsorbed CO probe molecule? Phys. Chem. Chem. Phys. 18, 22108–22121 (2016).
Zholobenko, V. L., Lei, G.-D., Carvill, B. T., Lerner, B. A. & Sachtler, W. M. H. Identification of isolated Pt atoms in H-mordenite. J. Chem. Soc. Faraday Trans. 90, 233–238 (1994).
Redhead, P. A. Thermal desorption of gases. Vacuum 12, 203–211 (1962).
Thang, H. V., Pacchioni, G., DeRita, L. & Christopher, P. Nature of stable single atom Pt catalysts dispersed on anatase TiO2. J. Catal. 367, 104–114 (2018).
Freund, H.-J., Meijer, G., Scheffler, M., Schlögl, R. & Wolf, M. CO oxidation as a prototypical reaction for heterogeneous processes. Angew. Chem. Int. Ed. 50, 10064–10094 (2011).
Bunluesin, T., Cordatos, H. & Gorte, R. J. Study of CO oxidation kinetics on Rh/ceria. J. Catal. 157, 222–226 (1995).
Allian, A. D. et al. Chemisorption of CO and mechanism of CO oxidation on supported platinum nanoclusters. J. Am. Chem. Soc. 133, 4498–4517 (2011).
Berlowitz, P. J., Peden, C. H. F. & Goodman, D. W. Kinetics of carbon monoxide oxidation on single-crystal palladium, platinum, and iridium. J. Phys. Chem. 92, 5213–5221 (1988).
Dai, S. et al. In situ atomic-scale observation of oxygen-driven core–shell formation in Pt3Co nanoparticles. Nat. Commun. 8, 204 (2017).
Matsubu, J. C. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Resasco, J., Dai, S., Graham, G., Pan, X. & Christopher, P. Combining in-situ transmission electron microscopy and infrared spectroscopy for understanding dynamic and atomic-scale features of supported metal catalysts. J. Phys. Chem. C 122, 25143–25157 (2018).
Crewe, A. V., Wall, J. & Langmore, J. Visibility of single atoms. Science 168, 1338–1340 (1970).
Diebold, U., Ruzycki, N., Herman, G. S. & Selloni, A. One step towards bridging the materials gap: surface studies of TiO2 anatase. Catal. Today 85, 93–100 (2003).
Schimka, L. et al. Accurate surface and adsorption energies from many-body perturbation theory. Nat. Mater. 9, 741–744 (2010).
Walsh, A., Sokol, A. A., Buckeridge, J., Scanlon, D. O. & Catlow, C. R. A. Oxidation states and ionicity. Nat. Mater. 17, 958–964 (2018).
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Hoffman, A. S., Singh, J. A., Bent, S. F. & Bare, S. R. In situ observation of phase changes of a silica-supported cobalt catalyst for the Fischer–Tropsch process by the development of a synchrotron-compatible in situ/operando powder X-ray diffraction cell. J. Synchrotron Radiat. 25, 1673–1682 (2018).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 78, 1396–1399 (1997).
Chen, H.-Y. T. Y. T., Tosoni, S. & Pacchioni, G. Adsorption of ruthenium atoms and clusters on anatase TiO2 and tetragonal ZrO2 (101) surfaces: a comparative DFT study. J. Phys. Chem. C 119, 10856–10868 (2015).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758 (1999).
Chen, H.-Y. T., Tosoni, S. & Pacchioni, G. A. DFT study of the acid–base properties of anatase TiO2 and tetragonal ZrO2 by adsorption of CO and CO2 probe molecules. Surf. Sci. 652, 163–171 (2016).
Acknowledgements
We acknowledge S. Hanukovich for his assistance with the microkinetic modelling in Supplementary Fig. 8 and the TPR experiment in Supplementary Fig. 18. P.C. acknowledges funding from National Science Foundation (NSF) CAREER grant number CBET-1823189. The UCSB MRL Shared Experimental Facilities are acknowledged for use of the inductively coupled plasma–optical emission spectrometry equipment and are supported by the MRSEC Program of the NSF under award no. DMR 1720256; a member of the NSF-funded Materials Research Facilities Network (www.mrfn.org). The work of H.V.T. and G.P. was supported by the Italian MIUR through the PRIN Project 2015K7FZLH SMARTNESS. Use of the Stanford Synchrotron Radiation Lightsource, SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515. Co-ACCESS is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences. TEM experiments was conducted using the facilities in the Irvine Materials Research Institute at the University of California-Irvine. The work at UC Irvine was supported by the National Science Foundation through the grant number DMR-1506535.
Author information
Authors and Affiliations
Contributions
L.D. developed the catalyst synthesis; L.D. and J.R. performed infrared characterization and catalytic measurements; S.D. performed and analysed TEM measurements with supervision by X.P. and G.W.G.; L.D., A.S.H., A.B. and I.R. performed the XAS supervised by S.R.B.; A.B. performed the XAFS analysis; H.V.T. performed the DFT calculations supervised by G.P.; I.R. performed the inductively coupled plasma–optical emission spectrometry measurements. All authors analysed and interpreted the results and contributed to the preparation of the manuscript. P.C. conceived the project and oversaw all portions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Extended materials and methods, text, Figs. 1–18, Tables 1–5, refs. 1–34
Rights and permissions
About this article
Cite this article
DeRita, L., Resasco, J., Dai, S. et al. Structural evolution of atomically dispersed Pt catalysts dictates reactivity. Nat. Mater. 18, 746–751 (2019). https://doi.org/10.1038/s41563-019-0349-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-019-0349-9
This article is cited by
-
Visualizing the structural evolution of individual active sites in MoS2 during electrocatalytic hydrogen evolution reaction
Nature Catalysis (2024)
-
Distribution of Pt single atom coordination environments on anatase TiO2 supports controls reactivity
Nature Communications (2024)
-
Photochemical tuning of dynamic defects for high-performance atomically dispersed catalysts
Nature Materials (2024)
-
Co-catalytic metal–support interactions in single-atom electrocatalysts
Nature Reviews Materials (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)