Abstract
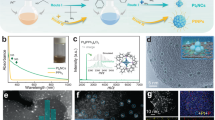
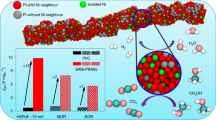
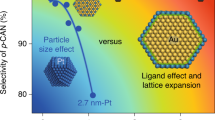
Bimetallic nanoparticles with tailored structures constitute a desirable model system for catalysts, as crucial factors such as geometric and electronic effects can be readily controlled by tailoring the structure and alloy bonding of the catalytic site. Here we report a facile colloidal method to prepare a series of platinum–gold (PtAu) nanoparticles with tailored surface structures and particle diameters on the order of 7 nm. Samples with low Pt content, particularly Pt4Au96, exhibited unprecedented electrocatalytic activity for the oxidation of formic acid. A high forward current density of 3.77 A mgPt−1 was observed for Pt4Au96, a value two orders of magnitude greater than those observed for core–shell structured Pt78Au22 and a commercial Pt nanocatalyst. Extensive structural characterization and theoretical density functional theory simulations of the best-performing catalysts revealed densely packed single-atom Pt surface sites surrounded by Au atoms, which suggests that their superior catalytic activity and selectivity could be attributed to the unique structural and alloy-bonding properties of these single-atomic-site catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the results of this work are available from the authors on reasonable request.
References
Chen, A. & Holt-Hindle, P. Platinum-based nanostructured materials: synthesis, properties, and applications. Chem. Rev. 110, 3767–3804 (2010).
Hunt, S. T. et al. Self-assembly of noble metal monolayers on transition metal carbide nanoparticle catalysts. Science 352, 974–978 (2016).
Yu, Y., Wang, X. & Lim, K. H. A DFT study on the adsorption of formic acid and its oxidized intermediates on (100) facets of Pt, Au, monolayer and decorated Pt@Au surfaces. Catal. Lett. 141, 1872–1882 (2011).
Wang, X., He, B., Hu, Z., Zeng, Z. & Han, S. Current advances in precious metal core–shell catalyst design. Sci. Technol. Adv. Mater. 15, 043502 (2014).
Liu, X., Wang, D. & Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 7, 448–466 (2012).
Liu, X., Wang, D. & Li, Y. Bimetallic nanocrystals: liquid-phase synthesis and catalytic applications. Adv. Mater. 23, 1044–1060 (2011).
Jiang, K., Zhang, H.-X., Zou, S. & Cai, W.-B. Electrocatalysis of formic acid on palladium and platinum surfaces: from fundamental mechanisms to fuel cell applications. Phys. Chem. Chem. Phys. 16, 20360–20376 (2014).
Liu, J. et al. Tackling CO poisoning with single atom alloy catalysts. J. Am. Chem. Soc. 138, 6396–6399 (2016).
Yuge, K., Koyama, Y., Kuwabara, A. & Tanaka, I. Surface design of alloy protection against CO-poisoning from first principles. J. Phys. Condens. Matter. 26, 355006 (2014).
Habrioux, A. et al. Structural and electrochemical studies of Au–Pt nanoalloys. Phys. Chem. Chem. Phys. 11, 3573–3579 (2009).
Ji, X. et al. Nanocrystalline intermetallics on mesoporous carbon for direct formic acid fuel cell anodes. Nat. Chem. 2, 286–293 (2010).
Zhang, H., Watanabe, T., Okumura, M., Haruta, M. & Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater. 11, 49–52 (2012).
Guo, S. et al. Nanocatalyst superior to Pt for oxygen reduction reactions: the case of core/shell Ag(Au)/CuPd nanoparticles. J. Am. Chem. Soc. 136, 15026–15033 (2014).
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Chen, G. et al. Interfacial effects in iron-nickel hydroxide–platinum nanoparticles enhance catalytic oxidation. Science 344, 495–499 (2014).
Roy, A. et al. Enhanced catalytic activity of Ag/Rh bimetallic nanomaterial: evidence of an ensemble effect. J. Phys. Chem. C 120, 5457–5467 (2016).
Prinz, J. et al. Ensemble effect evidenced by CO adsorption on the 3-fold PdGa surfaces. J. Phys. Chem. C 118, 12260–12265 (2014).
Zaera, F., Gellman, J. A. & Somorjai, G. A. Surface science studies of catalysis: classification of reactions. Acc. Chem. Res. 19, 24–31 (1986).
Ruff, M., Takehiro, N., Liu, P., Nørskov, J. K. & Behm, R. J. Size-specific chemistry on bimetallic surfaces: a combined experimental and theoretical study. ChemPhysChem 8, 2068–2071 (2007).
Sachtler, W. M. H. Chemisorption complexes on alloy surfaces. Catal. Rev. Sci. Eng. 14, 193–210 (1976).
Stevanović, S. et al. Insight into the effect of Sn on CO and formic acid oxidation at PtSn catalysts. J. Phys. Chem. C 118, 278–289 (2014).
Duchesne, P. N. & Zhang, P. Element-specific analysis of the growth mechanism, local structure, and electronic properties of Pt clusters formed on Ag nanoparticle surfaces. J. Phys. Chem. C 118, 21714–21721 (2014).
Yu, Y., Hu, Y., Liu, X., Deng, W. & Wang, X. The study of Pt@Au electrocatalyst based on Cu underpotential deposition and Pt redox replacement. Electrochim. Acta 54, 3092–3097 (2009).
Kim, J., Jung, C., Rhee, C. K. & Lim, T. Electrocatalytic oxidation of formic acid and methanol on Pt deposits on Au(111). Langmuir 23, 10831–10836 (2007).
Luo, J. et al. Phase properties of carbon-supported gold–platinum nanoparticles with different bimetallic compositions. Chem. Mater. 17, 3086–3091 (2005).
Atkins, P. & de Paula, J. Atkins' Physical Chemistry 8th edn, 1005–1006 (W.H. Freeman and Company, New York, 2006).
Wu, B. & Zheng, N. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8, 168–197 (2013).
Bligaard, T. et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004).
Zhong, W. & Zhang, D. New insight into the CO formation mechanism during formic acid oxidation on Pt(111). Catal. Commun. 29, 82–86 (2012).
Neurock, M., Janik, M. & Wieckowski, A. A first principles comparison of the mechanism and site requirements for the electrocatalytic oxidation of methanol and formic acid over Pt. Faraday Discuss. 140, 363–378 (2009).
Cuesta, A., Escudero, M., Lanova, B. & Baltruschat, H. Cyclic voltammetry, FTIRS, and DEMS study of the electrooxidation of carbon monoxide, formic acid, and methanol on cyanide-modified Pt(111) electrodes. Langmuir 25, 6500–6507 (2009).
Mason, M. Electronic structure of supported small metal clusters. Phys. Rev. B 27, 748–762 (1983).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Kraft, S., Stümpel, J., Becker, P. & Kuetgens, U. High resolution X-ray absorption spectroscopy with absolute energy calibration for the determination of absorption edge energies. Rev. Sci. Instrum. 67, 681–687 (1996).
Bearden, J. A. X-ray wavelengths. Rev. Mod. Phys. 39, 78–124 (1967).
Ressler, T. WinXAS: A program for X-ray absorption spectroscopy data analysis under MS-Windows. J. Synchrotron. Radiat. 5, 118–122 (1998).
Ankudinov, A. L., Ravel, B., Rehr, J. J. & Conradson, S. D. Real-space multiple-scattering calculation and interpretation of X-ray-absorption near-edge structure. Phys. Rev. B 58, 7565–7576 (1998).
Wagner, C. D. in Practical Surface Analysis (eds Briggs, D. & Seah, M. P.) 635–638 (Wiley, Hoboken, 1990).
Naumkin, A. V., Kraut-Vass, A., Gaarenstroom, S. W. & Powell, C. J. NIST X-ray Photoelectron Spectroscopy Database (NIST, 2012); http://srdata.nist.gov/xps/
Luo, S., Zhao, Y. & Truhlar, D. G. Improved CO adsorption energies, site preferences, and surface formation energies from a meta-generalized gradient approximation exchange–correlation functional, M06-L. J. Phys. Chem. Lett. 3, 2975–2979 (2012).
Acknowledgements
P.Z. acknowledges financial support from the NSERC Canada Discovery Grant and P.N.D. was funded by an NSERC CGS scholarship. Financial supports from European COST Action MP0903 ‘Nanoalloy’ (Z.Y.L.) and the US National Science Foundation DMR-1409396 (S.C.) are acknowledged. A.A. and Z.A. acknowledge the financial support by Deanship of Scientific Research, King Saud University. Part of this work was supported by a PCOSS Open Project Grant (Xiamen University) awarded to P.Z. and hosted by N.Z. DFT calculations were sponsored by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division and used resources of the National Energy Research Scientific Computing Center, a DOE Office of Science User Facility supported by the Office of Science of the U.S. Department of Energy under contract no. DE-AC02-05CH11231. This research used resources of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, and was supported by the US DOE under contract no. DE-AC02-06CH11357, and the Canadian Light Source and its funding partners. The Canadian Light Source is supported by the CFI, NSERC, NRC, CIHR, the University of Saskatchewan, the Government of Saskatchewan and Western Economic Diversification Canada. We are also grateful for the assistance of Z. Finfrock (CLS@APS) and Y. Hu (SXRMB@CLS) for synchrotron technical support, and L. Leonardo for the collection of additional EDX mapping in the JEOL-York Nanocenter using JEM-2200FS Cs-corrected (S)TEM operating at 200 keV.
Author information
Authors and Affiliations
Contributions
P.N.D. synthesized all the samples, conducted the XAS experiments and analysis, performed some of the electrochemical and TEM studies, and wrote the manuscript. P.Z. designed the project, coordinated the process of the work and supervised P.N.D. to conduct this research. Z.Y.L. and J.Y. performed the HAADF-STEM measurements and image analysis. C.P.D. performed the electrochemical experiments under the supervision of S.C. V.F. conducted the DFT calculations under the supervision of D.J. X.Z. contributed to the TEM measurements under the supervision of N.Z. A.A. and Z.A. also contributed to part of the TEM measurements. T.R. performed some of the XPS measurements at the Canadian Light Source.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–12, Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
Duchesne, P.N., Li, Z.Y., Deming, C.P. et al. Golden single-atomic-site platinum electrocatalysts. Nature Mater 17, 1033–1039 (2018). https://doi.org/10.1038/s41563-018-0167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0167-5
This article is cited by
-
Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation
Nature Chemistry (2024)
-
Co-catalytic metal–support interactions in single-atom electrocatalysts
Nature Reviews Materials (2024)
-
Atomically dispersed materials: Ideal catalysts in atomic era
Nano Research (2024)
-
Fundamentals and catalytic applications of single-atom alloys
Science China Materials (2024)
-
Embedding oxophilic rare-earth single atom in platinum nanoclusters for efficient hydrogen electro-oxidation
Nature Communications (2023)