Abstract
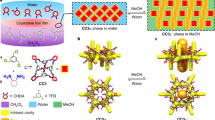
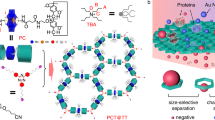
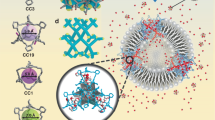
Protein pores are highly specific in binding to chiral substrates and in catalysing stereospecific reactions, because their active pockets are asymmetric and stereoselective1,2. Chiral binding materials from molecular-level pores with high specificity have not been achieved because of problems with pore deformation and blocking3. A promising solution is the self-assembly of single sheets where all pores are exposed to the environment, for example as metal–organic frameworks4, polymers5,6 or non-covalent aromatic networks7,8,9,10, but, typically, the pores are distant from the internal cavities with chirality. Here, we report the synthesis of homochiral porous nanosheets achieved by the 2D self-assembly of non-chiral macrocycles, with open/closed pore switching. Pore chirality is spontaneously induced by a twisted stack of dimeric macrocycles. The porous 2D structures can serve as enantiomer sieving membranes that exclusively capture a single enantiomer in a racemic mixture solution, with uptake capacity greater than 96%. Moreover, the entrapped guests inside the pores can be pumped out by pore closing triggered by external stimuli. This strategy could provide new opportunities for controlled molecule release, as well as for artificial cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gilson, M. K. & Zhou, H.-X. Calculation of protein–ligand binding affinities. Annu. Rev. Biophys. Biomol. Struct. 36, 21–42 (2007).
Stank, A., Kokh, D. B., Fuller, J. C. & Wade, R. C. Protein binding pocket dynamics. Acc. Chem. Res. 49, 809–815 (2016).
Cui, Y. et al. Metal–organic frameworks as platforms for functional materials. Acc. Chem. Res. 49, 483–493 (2016).
Peng, Y. et al. Metal–organic framework nanosheets as building blocks for molecular sieving membranes. Science 346, 1356–1359 (2014).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287–291 (2012).
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Colson, J. W. et al. Oriented 2D covalent organic framework thin films on single-layer graphene. Science 332, 228–231 (2011).
Dong, R. et al. Large-area, free-standing, two-dimensional supramolecular polymer single-layer sheets for highly efficient electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 54, 12058–12063 (2015).
Zhang, K. D. et al. Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 135, 17913–17918 (2013).
Yue, L. et al. Flexible single-layer ionic organic–inorganic frameworks towards precise nano-size separation. Nat. Commun. 7, 10742 (2016).
Iyoda, M. & Shimizu, H. Multifunctional π-expanded oligothiophene macrocycles. Chem. Soc. Rev. 44, 6411–6424 (2015).
Huang, Z. et al. Pulsating tubules from noncovalent macrocycles. Science 337, 1521–1526 (2012).
Percec, V. et al. Self-assembly of amphiphilic dendritic dipeptides into helical pores. Nature 430, 764–768 (2004).
Colson, J. W. & Dichtel, W. R. Rationally synthesized two-dimensional polymers. Nat. Chem. 5, 453–465 (2013).
Kim, Y. et al. Switchable nanoporous sheets by the aqueous self-assembly of aromatic macrobicycles. Angew. Chem. Int. Ed. 52, 6426–6429 (2013).
Shimizu, H. et al. Synthesis, structures, and photophysical properties of π-expanded oligothiophene 8-mers and their Saturn-like C60 complexes. J. Am. Chem. Soc. 137, 3877–3885 (2015).
Lee, S., Chen, C.-H. & Flood, A. H. A pentagonal cyanostar macrocycle with cyanostilbene CH donors binds anions and forms dialkylphosphate [3]rotaxanes. Nat. Chem. 5, 704–710 (2013).
Würthner, F., Kaiser, T. E. & Saha‐Möller, C. R. J-aggregates: from serendipitous discovery to supramolecular engineering of functional dye materials. Angew. Chem. Int. Ed. 50, 3376–3410 (2011).
Wang, Y., Xu, J., Wang, Y. & Chen, H. Emerging chirality in nanoscience. Chem. Soc. Rev. 42, 2930–2962 (2013).
Huang, K. & Szleifer, I. Design of multifunctional nanogate in response to multiple external stimuli using amphiphilic diblock copolymer. J. Am. Chem. Soc. 139, 6422–6430 (2017).
Nelson, J. C., Saven, J. G., Moore, J. S. & Wolynes, P. G. Solvophobically driven folding of nonbiological oligomers. Science 277, 1793–1796 (1997).
Chen, L. et al. Separation of rare gases and chiral molecules by selective binding in porous organic cages. Nat. Mater. 13, 954–960 (2014).
Zhang, G.-W. et al. Triptycene-based chiral macrocyclic hosts for highly enantioselective recognition of chiral guests containing a trimethylamino group. Angew. Chem. Int. Ed. 55, 5304–5308 (2016).
Hauser, A. W. et al. Functionalized graphene as a gatekeeper for chiral molecules: an alternative concept for chiral separation. Angew. Chem. Int. Ed. 53, 9957–9960 (2014).
Seo, J. S. et al. A homochiral metal–organic porous material for enantioselective separation and catalysis. Nature 404, 982–986 (2000).
Xie, R., Chu, L.-Y. & Deng, J.-G. Membranes and membrane processes for chiral resolution. Chem. Soc. Rev. 37, 1243–1263 (2008).
Kim, Y. et al. Open–closed switching of synthetic tubular pores. Nat. Commun. 6, 8650 (2015).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 21634005, 51473062 and 21574055) and Jilin University Funding (JLUSTIRT).
Author information
Authors and Affiliations
Contributions
B.S. synthesized molecules, measured spectroscopic data, carried out the uptake experiments. Y.K. performed chiral separation and molecular simulations, and supervised the research. Y.W. performed TEM experiments. H.W. carried out NMR experiments. J.K. performed X-ray experiments. X.L. measured electron diffraction. M.L. developed the concept, supervised the research and wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information
Supplementary Schemes 1–2, Supplementary Figures 1–34, Supplementary References 1–3
Rights and permissions
About this article
Cite this article
Sun, B., Kim, Y., Wang, Y. et al. Homochiral porous nanosheets for enantiomer sieving. Nature Mater 17, 599–604 (2018). https://doi.org/10.1038/s41563-018-0107-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0107-4
This article is cited by
-
Dendrimer-conjugated isotretinoin for controlled transdermal drug delivery
Journal of Nanobiotechnology (2023)
-
Enantiocontrolled macrocyclization by encapsulation of substrates in chiral capsules
Nature Synthesis (2023)
-
Bioinspired crowding directs supramolecular polymerisation
Nature Communications (2023)
-
Spiral fractal patterns via hierarchical assembly
Nano Research (2022)
-
Near quantitative synthesis of urea macrocycles enabled by bulky N-substituent
Nature Communications (2021)