Abstract
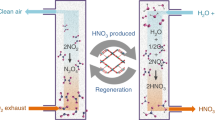
Nitrogen dioxide (NO2) is a major air pollutant causing significant environmental1,2 and health problems3,4. We report reversible adsorption of NO2 in a robust metal–organic framework. Under ambient conditions, MFM-300(Al) exhibits a reversible NO2 isotherm uptake of 14.1 mmol g−1, and, more importantly, exceptional selective removal of low-concentration NO2 (5,000 to <1 ppm) from gas mixtures. Complementary experiments reveal five types of supramolecular interaction that cooperatively bind both NO2 and N2O4 molecules within MFM-300(Al). We find that the in situ equilibrium 2NO2 ↔ N2O4 within the pores is pressure-independent, whereas ex situ this equilibrium is an exemplary pressure-dependent first-order process. The coexistence of helical monomer–dimer chains of NO2 in MFM-300(Al) could provide a foundation for the fundamental understanding of the chemical properties of guest molecules within porous hosts. This work may pave the way for the development of future capture and conversion technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lelieveld, J. et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 452, 737–740 (2008).
Edwards, P. M. et al. High winter ozone pollution from carbonyl photolysis in an oil and gas basin. Nature 514, 351–354 (2014).
Chen, Z., Wang, J., Ma, G. & Zhang, Y. China tackles the health effects of air pollution. Lancet 382, 1959–1960 (2013).
Feigin, V. L. et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet Neurol. 15, 913–924 (2013).
Rezaei, F., Rownaghi, A. A., Monjezi, S., Lively, R. P. & Jones, C. W. SOx/NOx removal from flue gas streams by solid adsorbents: a review of current challenges and future directions. Energy Fuels 29, 5467–5486 (2015).
Goupil, J. M., Hemidy, J. F. & Cornet, D. Adsorption of NO2 on modified Y zeolites. Zeolites 2, 47–50 (1982).
Levasseur, B., Ebrahim, A. M. & Bandosz, T. J. Role of Zr4+ cations in NO2 adsorption on Ce1−xZrxO2 mixed oxides at ambient conditions. Langmuir 27, 9379–9386 (2011).
Levasseur, B., Ebrahim, A. M. & Bandosz, T. J. Interactions of NO2 with amine-functionalized SBA-15: effects of synthesis route. Langmuir 28, 5703–5714 (2012).
Florent, M., Tocci, M. & Bandosz, T. J. NO2 adsorption at ambient temperature on urea-modified ordered mesoporous carbon. Carbon 63, 283–293 (2013).
Belhachemi, M., Jeguirim, M., Limousy, L. & Addoun, F. Comparison of NO2 removal using date pits activated carbon and modified commercialized activated carbon via different preparation methods: effect of porosity and surface chemistry. Chem. Eng. J. 253, 121–129 (2014).
Zhou, H. C. & Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 43, 5415 (2014).
Yang, S. et al. Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host. Nat. Chem. 4, 887–894 (2012).
Ebrahim, A. M. & Bandosz, T. J. Effect of amine modification on the properties of zirconium–carboxylic acid based materials and their applications as NO2 adsorbents at ambient conditions. Micropor. Mesopor. Mater. 188, 149–162 (2014).
Ebrahim, A. M., Levasseur, B. & Bandosz, T. J. Interactions of NO2 with Zr-based MOF: effects of the size of organic linkers on NO2 adsorption at ambient conditions. Langmuir 29, 168–174 (2013).
Peterson, G. W., Mahle, J. J., DeCoste, J. B., Gordon, W. O. & Rossin, J. A. Extraordinary NO2 removal by the metal–organic framework UiO-66-NH2. Angew. Chem. Int. Ed. 55, 6235–6238 (2016).
DeCoste, J. B., Demasky, T. J., Katz, M. J., Farha, O. K. & Hupp, J. T. A UiO-66 analogue with uncoordinated carboxylic acids for the broad-spectrum removal of toxic chemicals. New J. Chem. 39, 2396–2399 (2015).
Ebrahim, A. M. & Bandosz, T. J. Ce(III) doped Zr-based MOFs as excellent NO2 adsorbents at ambient conditions. ACS Appl. Mater. Interfaces 5, 10565–10573 (2013).
Levasseur, B., Petit, C. & Bandosz, T. J. Reactive adsorption of NO2 on copper-based metal−organic framework and graphite oxide/metal−organic framework composites. ACS Appl. Mater. Interfaces 2, 3606–3613 (2010).
Petit, C., Levasseur, B., Mendoza, B. & Bandosz, T. J. Reactive adsorption of acidic gases on MOF/graphite oxide composites. Micropor. Mesopor. Mater. 154, 107–112 (2012).
Kvick, Å., McMullan, R. K. & Newton, M. D. The structure of dinitrogen tetroxide N2O4: neutron diffraction study at 100, 60, and 20 K and ab initio theoretical calculations. J. Chem. Phys. 76, 3754–3761 (1982).
Kachi-Terajima, C., Akatsuka, T., Kohbara, M. A. & Takamizawa, S. Structural and magnetic study of N2, NO, NO2, and SO2 adsorbed within a flexible single-crystal adsorbent of [Rh2(bza)4(pyz)]n. Chem. Asian J. 2, 40–50 (2007).
Wang, X., Hanson, J. C., Kwak, J. H., Szanyi, J. & Peden, C. H. F. Cation movements during dehydration and NO2 desorption in a Ba–Y, FAU zeolite: an in situ time-resolved X-ray diffraction study. J. Phys. Chem. C 117, 3915–3922 (2013).
Guttman, A. Absolute infrared intensity measurements on nitrogen dioxide and dinitrogen tetroxide. J. Quant. Spectrosc. Radiat. Transf. 2, 1–15 (1962).
Shlotani, M. & Freed, J. H. ESR studies of NO2 adsorbed on surfaces: analysis of motional dynamics. J. Phys. Chem. 85, 3873–3883 (1981).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Giauque, W. F. & Kemp, J. D. The entropies of nitrogen tetroxide and nitrogen dioxide; the heat capacity from 15°K to the boiling point; the heat of vaporization and vapor pressure; the equilibria N2O4 = 2NO2 = 2NO + O2. J. Chem. Phys. 6, 40–52 (1938).
Cooper, A. I. & Poliakoff, M. High-pressure reactions in polyethylene films, a new development in matrix isolation. The photochemical reaction of Fe(CO)5 with N2 and the thermal reaction of Fe(CO)4(N2) with H2. Chem. Phys. Lett. 212, 611–616 (1993).
Clark, S. J. et al. First principles methods using CASTEP. Z. Krist. 220, 567–570 (2005).
Ramirez-Cuesta, A. J. aCLIMAX 4.0.1, The new version of the software for analyzing and interpreting INS spectra. Comput. Phys. Commun. 157, 226–238 (2004).
Rietveld, H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969).
Schweiger, A. and Jeschke, G. Principles of Pulse Electron Paramagnetic Resonance (Oxford Univ. Press, Oxford, 2001).
Stoll, S. & Britt, R. D. General and efficient simulation of pulse EPR spectra. Phys. Chem. Chem. Phys. 11, 6614–6625 (2009).
Acknowledgements
We thank the EPSRC (EP/I011870), the ERC (AdG 226593) and the Universities of Manchester and Nottingham for funding. We thank the EPSRC for funding of the EPSRC National Service for EPR Spectroscopy at Manchester. We are especially grateful to ORNL and the ESRF for access to the Beamlines VISION and ID22, respectively. We thank C. Dejoie for help at Beamline ID22 at the ESRF. The computing resources were made available through the VirtuES and the ICE-MAN projects, funded by the Laboratory Directed Research and Development program at ORNL. A.M.S. thanks the Russian Science Foundation (grant no. 17-73-10320) and the Royal Society of Chemistry for funding. M.S. acknowledges the Russian Ministry of Science and Education for the award of a Russian Megagrant (14.Z50.31.0006).
Author information
Authors and Affiliations
Contributions
X.H., H.G.W.G. and L.B. performed syntheses, characterization of MOF samples and measurements of adsorption isotherms. X.H. carried out measurements and analysis of the breakthrough data. K.M.T. performed analysis of isotherms. S.Y., J.S. and C.D. were responsible for collection and analysis of synchrotron PXRD data. S.Y., Y.C., L.L.D. and A.J.R.-C. were responsible for collection and analysis of neutron scattering data. A.J.D. and M.W.G. were responsible for collection and analysis of infrared data. X.H., A.M.S., F.T. and E.J.L.M. were responsible for collection and analysis of EPR data. S.Y. and M.S. were responsible for the overall direction of the project and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Sections 1–14, Supplementary Figures 1–36, Supplementary Tables 1–7, Supplementary References 1–18
Rights and permissions
About this article
Cite this article
Han, X., Godfrey, H.G.W., Briggs, L. et al. Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework. Nature Mater 17, 691–696 (2018). https://doi.org/10.1038/s41563-018-0104-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-018-0104-7
This article is cited by
-
Synthesis of sawdust-based porous carbon using Box–Behnken design for NO2 adsorption: modeling, optimization, and study of interaction effects
Chemical Papers (2023)
-
Direct photo-oxidation of methane to methanol over a mono-iron hydroxyl site
Nature Materials (2022)
-
A Novel Artificial Neuron-Like Gas Sensor Constructed from CuS Quantum Dots/Bi2S3 Nanosheets
Nano-Micro Letters (2022)
-
Donut-like MOFs of copper/nicotinic acid and composite hydrogels with superior bioactivity for rh-bFGF delivering and skin wound healing
Journal of Nanobiotechnology (2021)
-
Computing inelastic neutron scattering spectra from molecular dynamics trajectories
Scientific Reports (2021)